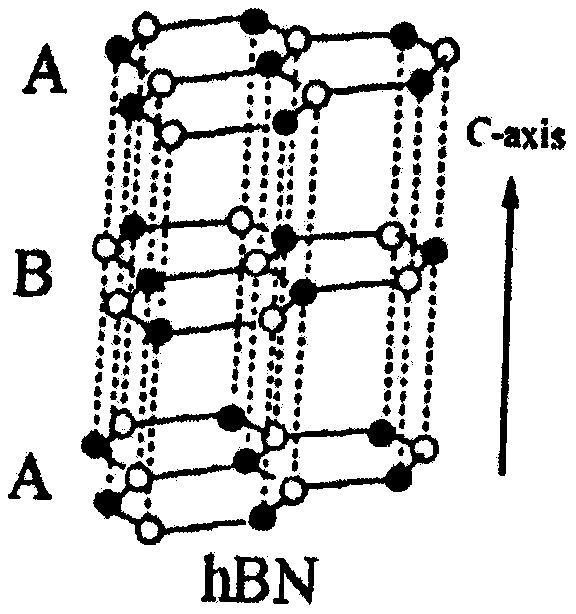
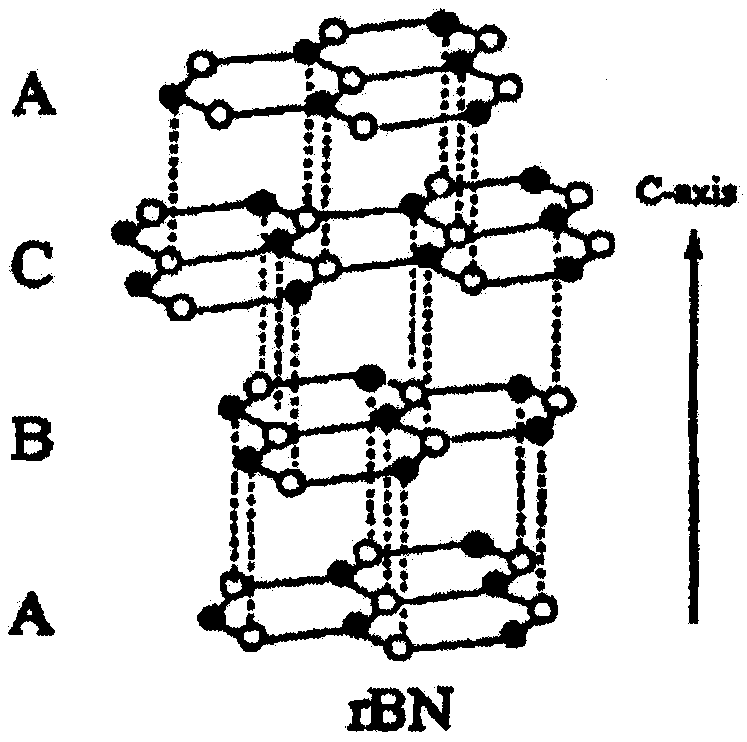
Preparation method of two-dimensional hydroxylated boron nitride
A technology of boron nitride and hydroxylation, applied in chemical instruments and methods, nitrogen compounds, inorganic chemistry, etc., can solve the problems of lack of active groups, limited improvement effect, poor dispersion, etc., and achieve simple preparation, improved dispersion performance, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Put sodium amide, ammonium fluoroborate and nickel with a molar ratio of 0.225:0.0283:0.0139 into the autoclave, flush with nitrogen as a protective gas, and rise from room temperature to 600°C at a rate of 10°C / min. Maintain at 600°C for 14h.
[0022] (2) After washing the product with concentrated hydrochloric acid and deionized water, dry it in a vacuum oven for subsequent use.
[0023] (3) Trigonal boron nitride and ammonium bicarbonate with a mass ratio of 1:2 were mixed and put into a ball mill jar for ball milling for 6 hours.
[0024] (4) The product is placed in an environment of 600°C, and the ammonium bicarbonate is decomposed into gas to obtain two-dimensional boron nitride powder.
[0025] (5) Two-dimensional boron nitride powder and 5 mmol / mL sodium hydroxide aqueous solution were mixed and added into a hydrothermal kettle, and heated at 120° C. for 12 hours to obtain hydroxylated boron nitride.
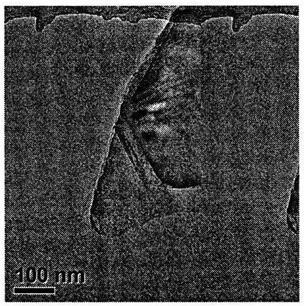
[0026] image 3 The transmission electron microscope...
Embodiment 2
[0028] (1) Put sodium amide, ammonium fluoroborate and nickel with a molar ratio of 0.225:0.0283:0.0139 into the autoclave, flush with nitrogen as a protective gas, and rise from room temperature to 600°C at a rate of 10°C / min. Maintain at 600°C for 18h.
[0029] (2) After washing the product with concentrated hydrochloric acid and deionized water, dry it in a vacuum oven for subsequent use.
[0030] (3) Trigonal boron nitride and ammonium bicarbonate with a mass ratio of 1:2 were mixed and put into a ball mill jar for ball milling for 6 hours.
[0031] (4) The product is placed in an environment of 600°C, and the ammonium bicarbonate is decomposed into gas to obtain two-dimensional boron nitride powder.
[0032] (5) Two-dimensional boron nitride powder and 5 mmol / mL sodium hydroxide aqueous solution were mixed and added into a hydrothermal kettle, and heated at 120° C. for 12 hours to obtain hydroxylated boron nitride.
Embodiment 3
[0034] (1) Put sodium amide, ammonium fluoroborate and nickel with a molar ratio of 0.225:0.0283:0.0139 into the autoclave, flush with nitrogen as a protective gas, and rise from room temperature to 600°C at a rate of 10°C / min. Maintain at 600°C for 14h.
[0035] (2) After washing the product with concentrated hydrochloric acid and deionized water, dry it in a vacuum oven for subsequent use.
[0036] (3) Trigonal boron nitride and ammonium bicarbonate with a mass ratio of 1:10 were mixed and put into a ball mill jar for ball milling for 6 hours.
[0037] (4) The product is placed in an environment of 600°C, and the ammonium bicarbonate is decomposed into gas to obtain two-dimensional boron nitride powder.
[0038] (5) Two-dimensional boron nitride powder and 5 mmol / mL sodium hydroxide aqueous solution were mixed and added into a hydrothermal kettle, and heated at 120° C. for 12 hours to obtain hydroxylated boron nitride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com