Fluorescence detection test strip for simultaneously detecting four types of pathogenic bacteria, and preparation method and application thereof
A technology for fluorescence detection and pathogenic bacteria, which is applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of high cost, low detection efficiency, low sensitivity, etc., and achieve the effect of low cost, low detection cost, and improved detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 A kind of fluorescence detection test strip that detects 4 kinds of pathogenic bacteria simultaneously
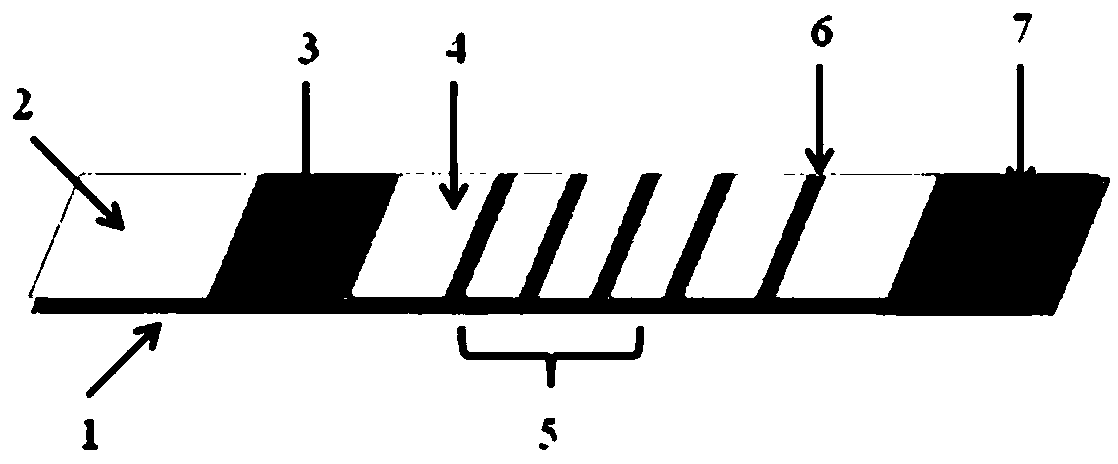
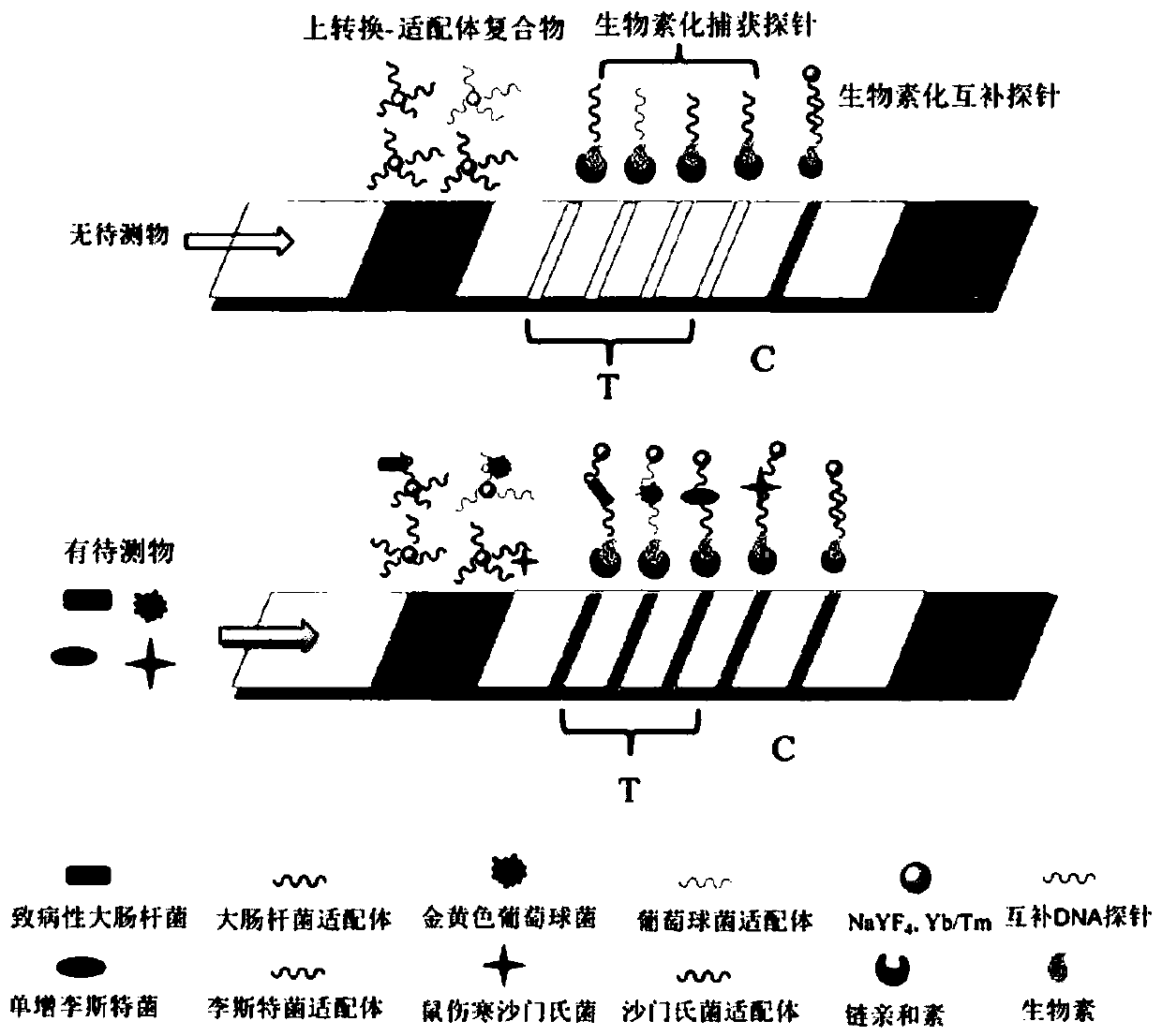
[0041] This embodiment provides a fluorescence detection test strip for four kinds of pathogenic bacteria based on upconversion nanomarker-aptamer recognition, including a PVC base plate 1, a sample pad 2, and an aptamer-functionalized upconversion nanoprobe binding pad 3 , a nitrocellulose membrane 4 and an absorbent pad 7; four detection lines 5 and quality control lines 6 are sequentially arranged on the nitrocellulose membrane 4; the adjacent pastings are connected and stacked.
[0042] The binding pad (3) is coated with an upconversion nanoparticle-pathogen first nucleic acid aptamer complex; the upconversion nanoparticle is an avidin-modified upconversion nanomaterial; the first nucleic acid The aptamer is capable of specifically recognizing Escherichia coli O157:H7, Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes, and its...
Embodiment 2
[0060] Embodiment 2: A kind of preparation method of the fluorescence detection test strip that detects 4 kinds of pathogenic bacteria simultaneously
[0061] The preparation method of this test strip mainly comprises the following steps:
[0062] (1) Avidin-coupled amino-functionalized upconversion nanomaterials
[0063] Quantitatively weigh 10mg NaYF 4 , YB / Tm up-converting nanomaterials were dispersed in 5 mL of PBS (0.01 mol / L), ultrasonically dispersed for 15 min, and 1.25 mL of 25% glutaraldehyde solution was added. Then, the mixed solution was shaken and incubated at room temperature for 2 hours, centrifuged, and the supernatant was discarded to obtain a solid powder. Wash three times with 0.01mol / L PBS to remove excess glutaraldehyde, resuspend the powder in 4mL0.01mol / L PBS, ultrasonically disperse, add 1mg / mL avidin, shake and incubate at room temperature for 12h, centrifuge, discard Clear, wash the material several times, and dry the obtained precipitate at 37°C ...
Embodiment 3
[0074] Embodiment 3: the usage method of the fluorescence detection test strip that detects 4 kinds of pathogenic bacteria simultaneously
[0075] (1) Test strip detection
[0076] Take out the prepared test strip, insert the end of the glass fiber membrane into the sample solution to be tested, take it out after waiting for 10 minutes, and detect the fluorescent signals of T line and C line at 980nm excitation and 452nm emission wavelength.
[0077] (2) Result judgment
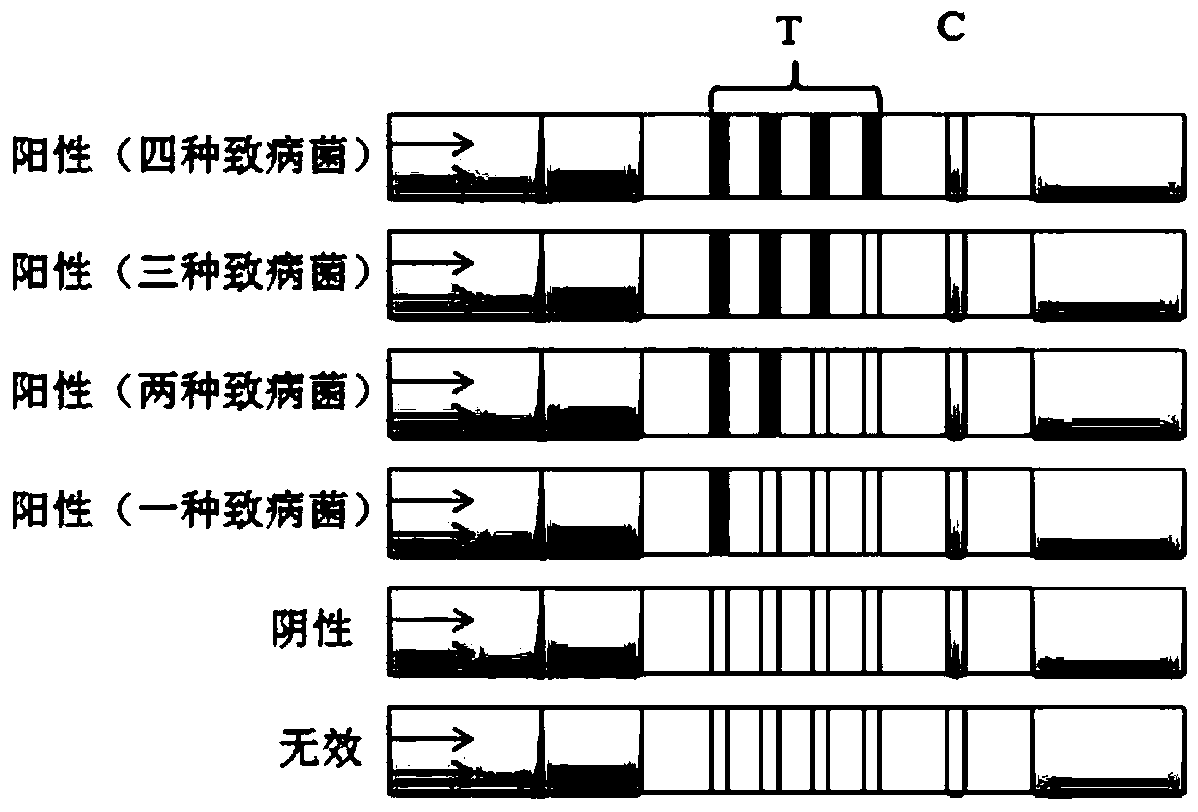
[0078] Positive: If any one or more of the quality control line 6 and the detection line 5 develop color, it means that the target bacteria are contained in the sample to be tested, and the sample is positive. For the result judgment, see image 3 shown.
[0079] Negative: If the test line 5 does not develop color, and only the quality control line 6 develops color, it means that the sample to be tested does not contain the four food-borne pathogenic bacteria, and the sample to be tested is negative. See ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com