Method for directly producing 1,2-pentanediol through furfural hydrogenation
A technology of pentanediol and furfural, which is applied in the field of direct production of 1,2-pentanediol by hydrogenation of furfural, can solve the problems of high equipment and operation requirements, expensive preparation cost of noble metal catalysts, high reaction pressure of Cu-based catalysts, etc. Low cost, low catalyst cost, simple and fast energy consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
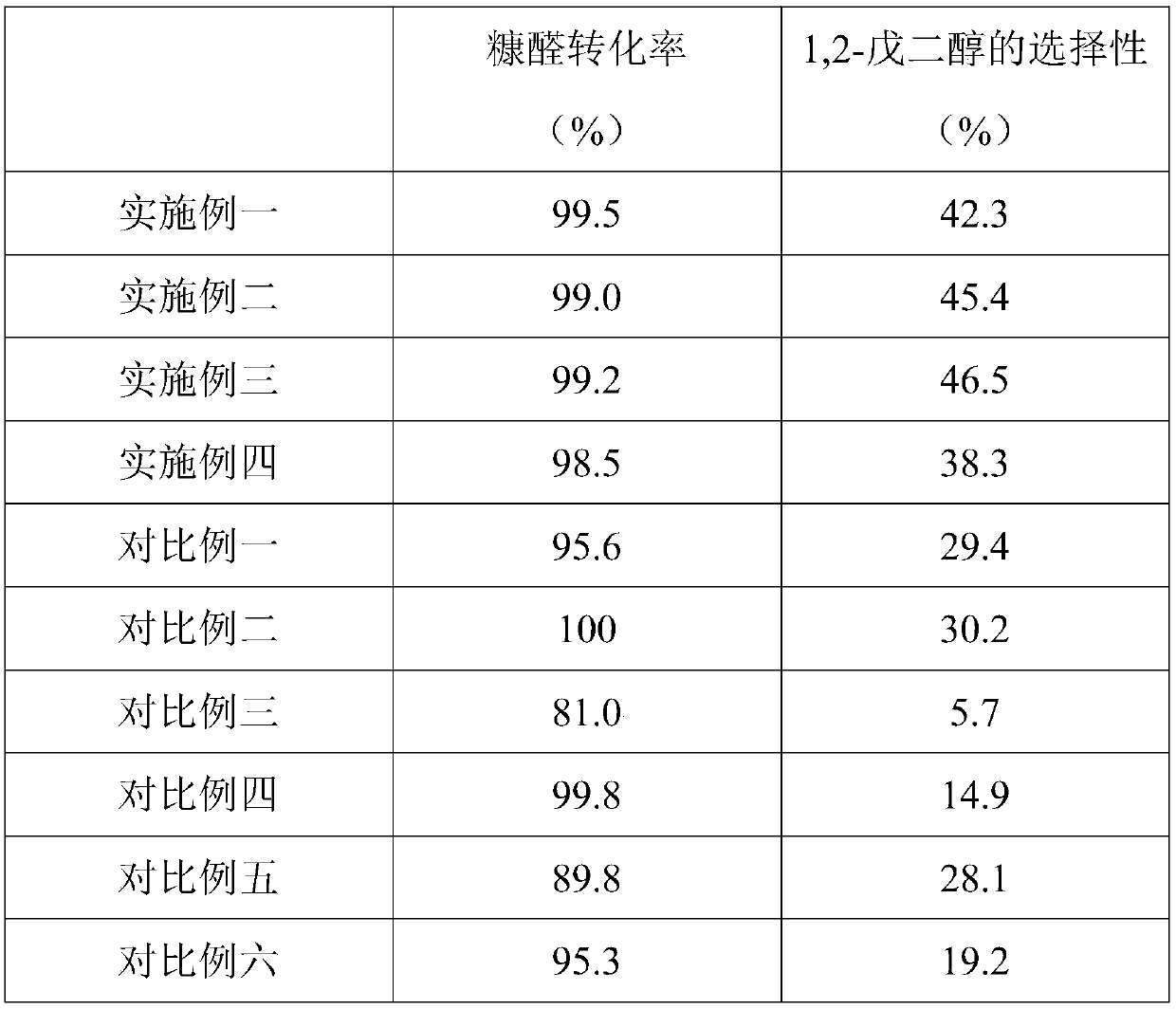
[0031] Embodiment 1: A kind of method of furfural hydrogenation direct production 1,2-pentanediol
[0032] Its production method is: add 11.9g of cobalt chloride hexahydrate and 150mL of water into a 500mL round bottom flask, stir to dissolve and place it in an ice-water bath, and add 6.8g of KBH 4 Dissolve it in 150mL of water and place it in a dropping funnel. Under the protection of a nitrogen atmosphere, slowly add it dropwise to the above round bottom flask, the reaction proceeds rapidly, a large amount of gas is released, and Co-B precipitates are formed. When no gas is generated, filter the solid product, wash the solid product with deionized water and absolute ethanol for 3-5 times, and finally store the sample in absolute ethanol for later use. Put 0.2g of Co-B amorphous alloy catalyst, 0.5g of furfural, and 10.0mL of ethanol into a 50mL stainless steel reactor with polytetrafluoroethylene, and react at a temperature of 140°C and a pressure of 1.5MPa for 12h.
Embodiment 2
[0033] Embodiment two: a kind of method of furfural hydrogenation direct production 1,2-pentanediol
[0034] Its production method is: add 5.95g of cobalt chloride hexahydrate, 5.95g of nickel chloride hexahydrate and 150mL of water into a 500mL round-bottomed flask, stir to dissolve and put it in an ice-water bath, and add 6.75g of KBH 4 Dissolve in 150mL of water and place it in a dropping funnel. Under the protection of a nitrogen atmosphere, slowly add it dropwise to the above round bottom flask. The reaction proceeds rapidly, a large amount of gas is released, and a black precipitate of Co-Ni-B is formed. When no gas is generated, filter the solid product, wash the solid product with deionized water and absolute ethanol for 3-5 times, and finally store the sample in absolute ethanol for later use. Put 0.2g Co-Ni-B amorphous alloy catalyst, 0.5g furfural, and 10.0mL ethanol into a 50mL stainless steel reactor with polytetrafluoroethylene, and react at a temperature of 160°...
Embodiment 3
[0035] Embodiment three: a kind of method of furfural hydrogenation direct production 1,2-pentanediol
[0036]Its production method is as follows: add 11.9g of cobalt chloride hexahydrate and 150mL of water into a 500mL round bottom flask, stir to dissolve and place it in an ice-water bath, and add 4.73g of NaBH 4 and 2.75g NaH 2 PO 2 Dissolve it in 150mL of water and place it in a dropping funnel. Under the protection of nitrogen atmosphere, slowly add it dropwise into the above round bottom flask, the reaction proceeds rapidly, a large amount of gas is released, and a black precipitate of Co-P-B is formed. When no gas is generated, filter the solid product, wash the solid product with deionized water and absolute ethanol for 3-5 times, and finally store the sample in isopropanol for later use. Put 0.2g Co-P-B amorphous alloy catalyst, 0.5g furfural, and 10.0mL isopropanol into a 50mL stainless steel reactor with polytetrafluoroethylene, and react at a temperature of 120°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com