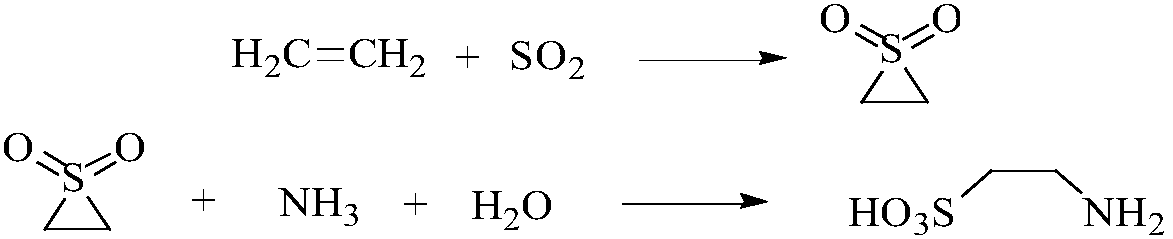Method for preparing taurine
A technology of taurine and ethyl orthosilicate, applied in the preparation of sulfonic acid, organic chemistry and other directions, can solve the problems of long reaction period, potential safety hazards and high cost, and achieve the effects of short production period, convenient operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Dissolve 4.29g of cetyl ammonium bromide in 50ml of 10wt% ammonia water, stir to dissolve and add NaAlO 2 0.058g, under strong stirring, slowly add 12.3g of ethyl orthosilicate dropwise, and stir for 3 hours to obtain a sol-gel solution, crystallize at 120°C to 125°C for 7 days, filter out the solid, wash until neutral, and then Dry at 105°C-110°C, bake at 800-850°C for 8 hours, press into tablets, grind, and sieve, and take about 8.3g of the 40-70 mesh particle catalyst Al-MCM-41 for use.
[0037] to N 2 After replacing the autoclave, add 95.2g of water and 0.3g of Al-MCM-41 molecular sieve catalyst into the autoclave, add 56.0g of ethylene into the autoclave, pump in 134.4g of sulfur dioxide, start stirring, and heat up to 75°C. 2 The pressure was charged to 7Mpa, the temperature was lowered after 1 hour of reaction, and 159.2 g of 1,1-dioxyethylene thioether was quantified by NMR. The conversion rate of ethylene in the addition reaction was 99.2%, and the selectivi...
Embodiment 2
[0043] N 2 After replacing the autoclave, add 78.6g of water into the autoclave, 0.50g of the Al-MCM-41 type molecular sieve catalyst in Example 1, add 42.0g of ethylene into the autoclave, pump 115.0g of sulfur dioxide into the autoclave, start stirring, and heat up to 80°C, N 2 The pressure was charged to 7.5Mpa, the temperature was lowered after 40 minutes of reaction, and 107.7g of 1,1-dioxyepithione was quantified by NMR. The conversion rate of added ethylene was 95.9%, and the selectivity was 81.3%.
[0044] Transfer the addition reaction solution to the ammonolysis reactor, N 2 Replaced three times, added liquid ammonia 109.4g (6.44mol) and raised the temperature to 120°C. 2 Adjust the pressure to 10Mpa, and cool down after reacting for 50min. LC external standard quantitative taurine 130.2g. The conversion rate of 1,1-dioxyethylene thioether was 99.2%, and the selectivity was 89.7%.
Embodiment 3
[0046] Dissolve cetyl ammonium bromide 3.2g with 10wt% ammonia water 50ml, add Ni(NO 3 ) 2 0.07g, under strong stirring, slowly add 6.1g of ethyl orthosilicate dropwise, and stir for 3 hours to obtain a sol-gel solution, crystallize at 120°C to 125°C for 6 days, filter out the solid, wash until neutral, and then Dry at 105°C-110°C, bake at 800-850°C for 7 hours, press into tablets, grind and sieve, and take 3.4g of Ni-MCM-41 as a 40-70 mesh particle catalyst for use.
[0047] N 2 After replacing the autoclave, add 275g of water and 1.6g of Ni-MCM-41 molecular sieve catalyst into the autoclave, add 156.8g of ethylene into the autoclave, pump 394.2g of sulfur dioxide into the autoclave, start stirring, and heat up to 80°C. 2 Increase the pressure to 8Mpa, cool down after 40 minutes of reaction, and detect 463.7g of 1,1-dioxyethylene thioether. The conversion rate of added ethylene was 97.7%, and the selectivity was 92.1%.
[0048] Transfer the addition reaction solution to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com