Catalyst for nitrous oxide decomposition and preparation method of catalyst
A nitrous oxide and catalyst technology, which is applied in the field of nitrous oxide decomposition catalyst and its preparation, can solve the problems of easy peeling off of the coating, high cost, complicated operation, etc., and achieve the effect of firm coating and short preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
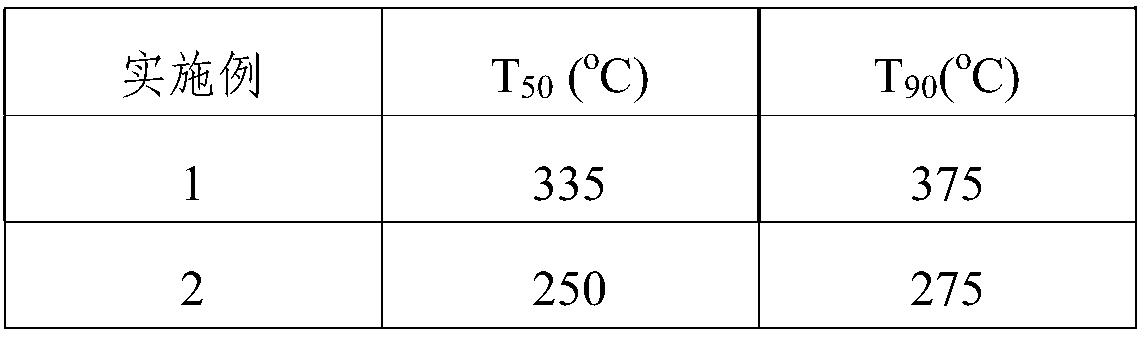
Examples
Embodiment 1
[0040] This embodiment provides a catalyst preparation method for the decomposition of nitrous oxide, comprising the following steps:
[0041] 1) Use ethyl orthosilicate as the silicon source, aluminum isopropoxide as the aluminum source, and N,N,N-trimethyl-1-adamantyl ammonium hydroxide as the template; press 30SiO 2 :Al 2 o 3 :24C 13 h 25 NO:120H 2 Molar ratio of O, adding silicon source, aluminum source, N,N,N-trimethyl-1-adamantyl ammonium hydroxide to water, and adjusting the pH value to 13-14, and preparing a molecular sieve mother liquor; Said molecular sieve mother liquor emulsification 2h;
[0042] 2) At a temperature of 80°C, soak honeycomb cordierite (specification: 400cpsi, 6.5mil; cut into square blocks of 10mm×10mm×10mm size) into a 15% nitric acid solution for 2 hours, and then use Wash with deionized water until neutral, then dry;
[0043] 3) In a hydrothermal reaction kettle, immerse the dried cordierite in step 2) into the molecular sieve mother liquo...
Embodiment 2
[0046] This embodiment provides a catalyst preparation method for the decomposition of nitrous oxide, comprising the following steps:
[0047] 1) Use ethyl orthosilicate as the silicon source, aluminum isopropoxide as the aluminum source, and N,N,N-trimethyl-1-adamantyl ammonium hydroxide as the template; press 30SiO 2 :Al 2 o 3 :24C 13 h 25 NO:120H 2Molar ratio of O, adding silicon source, aluminum source, N,N,N-trimethyl-1-adamantyl ammonium hydroxide to water, and adjusting the pH value to 13-14, and preparing a molecular sieve mother liquor; Said molecular sieve mother liquor emulsification 2h;
[0048] 2) Soak the honeycomb cordierite (specification: 600cpsi, 4mil; cut into square blocks with a size of 10mm×10mm×10mm) in 20% nitric acid solution for 3 hours at a temperature of 85°C, and then use Wash with ionized water until neutral, then dry;
[0049] 3) In a hydrothermal reaction kettle, immerse the dried cordierite in step 2) into the molecular sieve mother liqu...
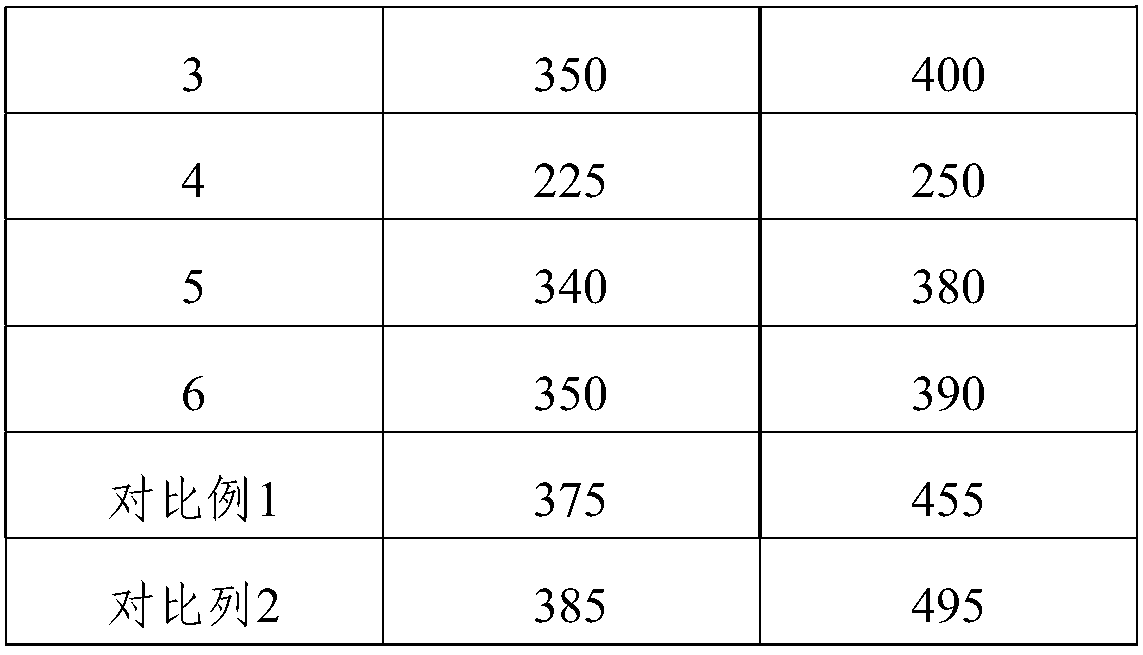
Embodiment 3
[0052] This embodiment provides a catalyst preparation method for the decomposition of nitrous oxide, comprising the following steps:
[0053] 1) Use ethyl orthosilicate as the silicon source, aluminum isopropoxide as the aluminum source, and N,N,N-trimethyl-1-adamantyl ammonium hydroxide as the template; press 30SiO 2 :Al 2 o 3 :24C 13 h 25 NO:120H 2 Molar ratio of O, adding silicon source, aluminum source, N,N,N-trimethyl-1-adamantyl ammonium hydroxide to water, and adjusting the pH value to 13-14, and preparing a molecular sieve mother liquor; Said molecular sieve mother liquor emulsification 2h;
[0054] 2) Soak honeycomb cordierite (specification: 600cpsi, 4mil; cut into square blocks with a size of 10mm×10mm×10mm) into a nitric acid solution with a concentration of 20% for 3 hours at a temperature of 85°C, Then wash with deionized water until neutral, then dry;
[0055] 3) In a hydrothermal reaction kettle, immerse the dried cordierite in step 2) into the molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hole density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com