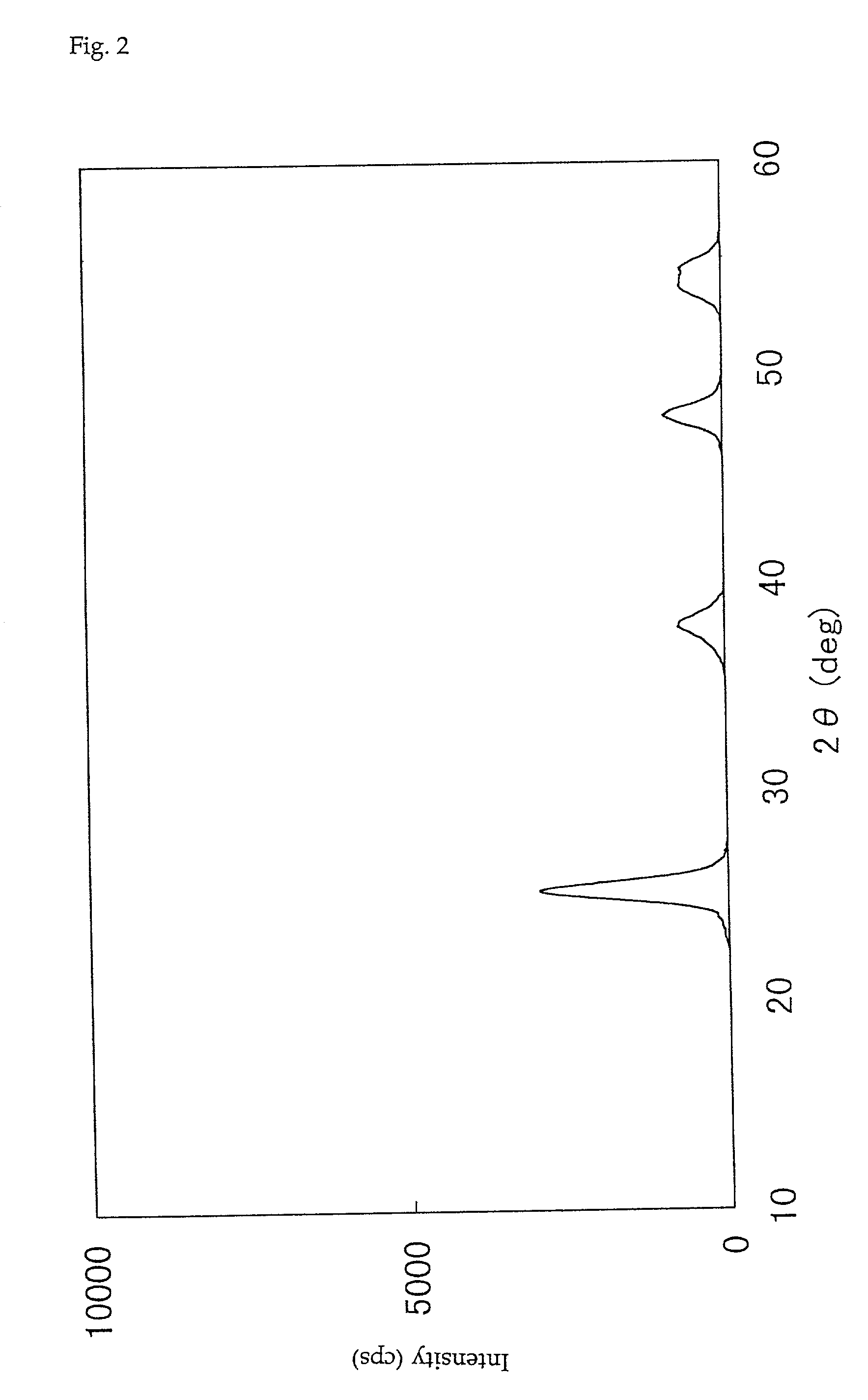
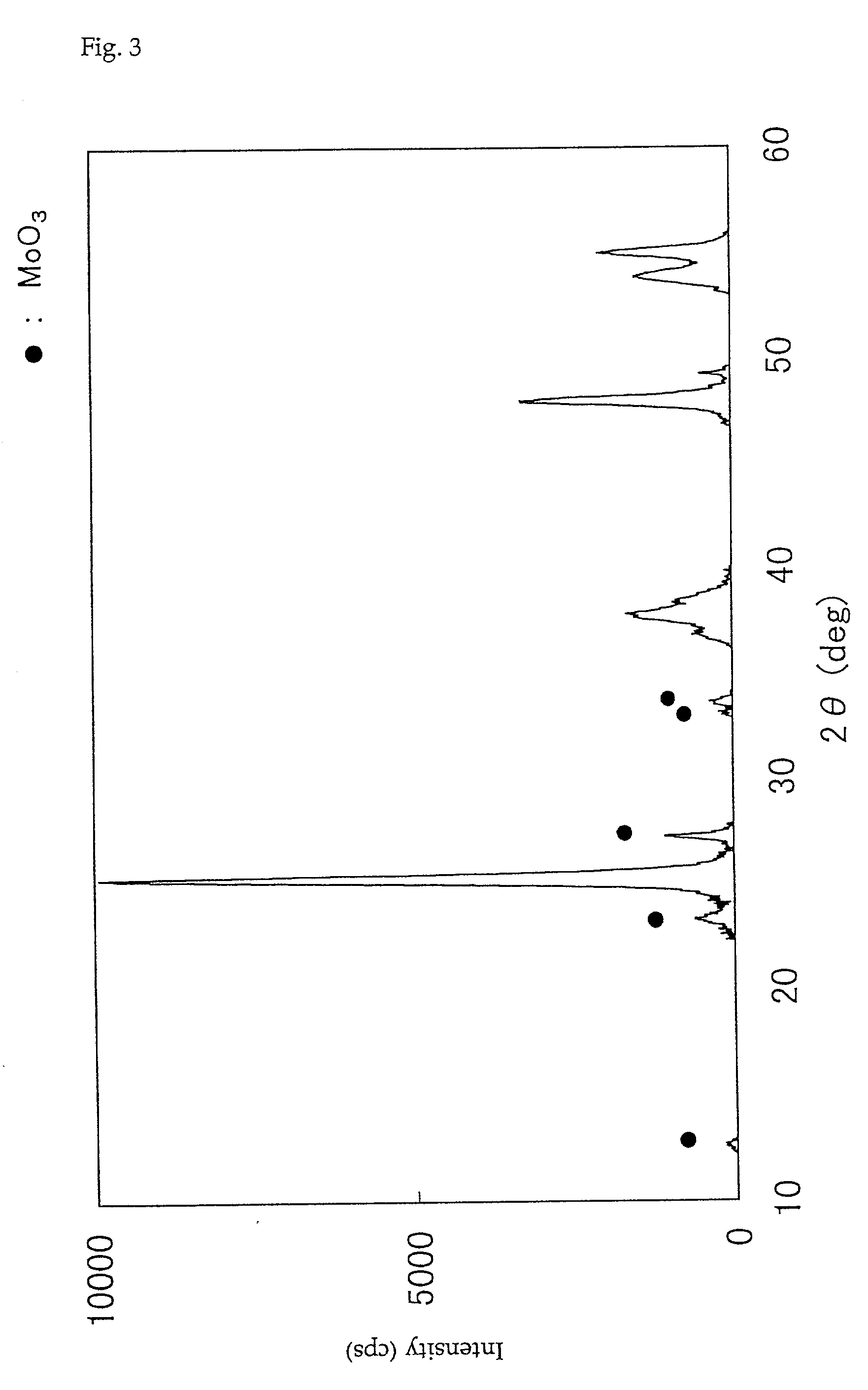
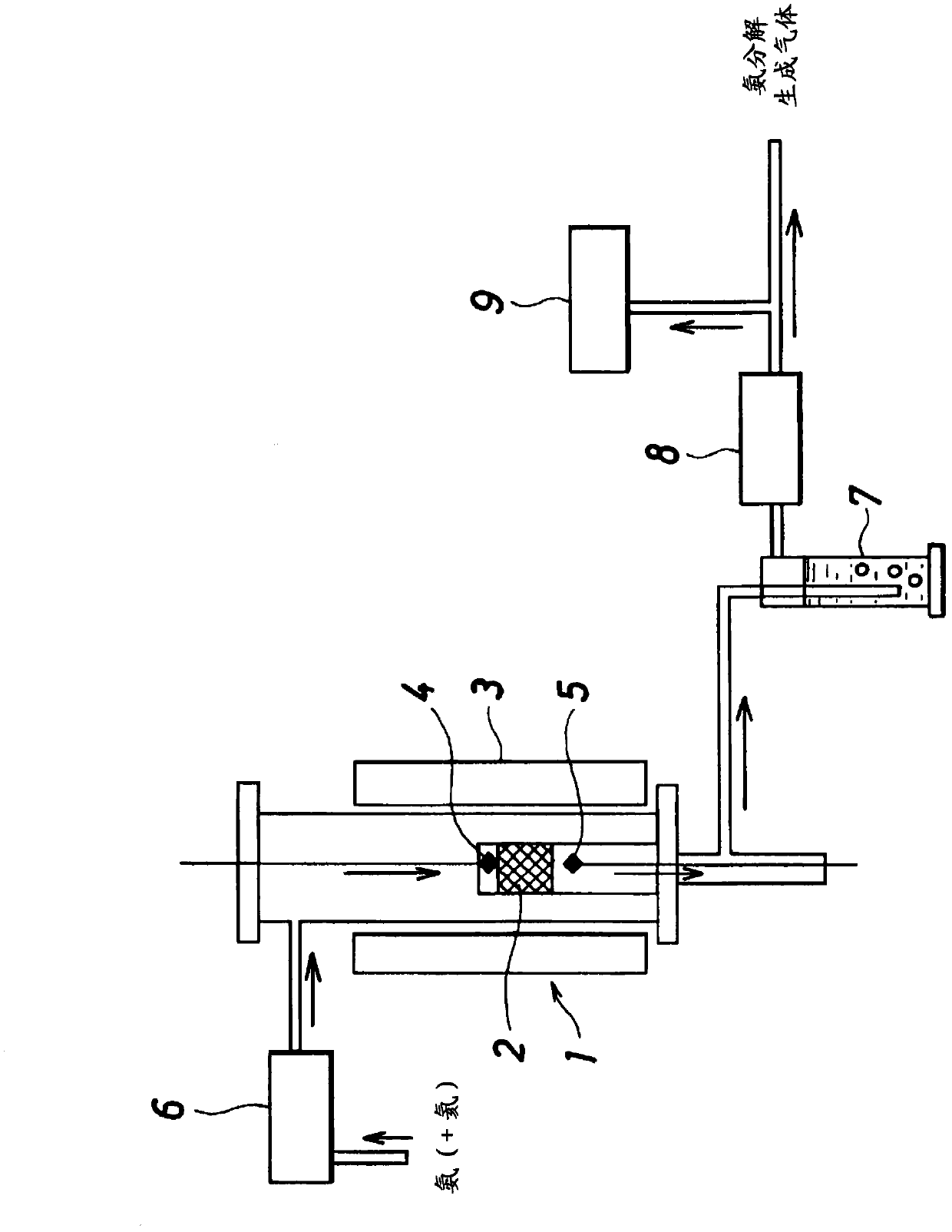
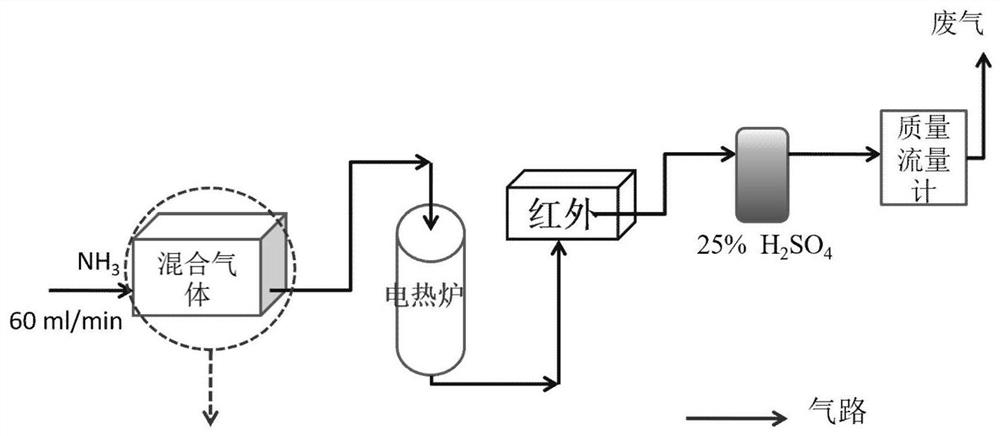
Patents
Literature
46results about How to "High decomposition activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composite nano-photo-catalyst used for purifying air
InactiveCN1618516AExtended service lifeHigh activityDispersed particle separationCatalyst activation/preparationBenzenePhoto catalytic
A composite nano-photocatalyst for cleaning air is composed of anatase-type TiO2, and the (15-25)-nm Fe2O3 and V2O5. It can effectively decompose the formaldehyde, benzene and ammonia in air under irradiation of light.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
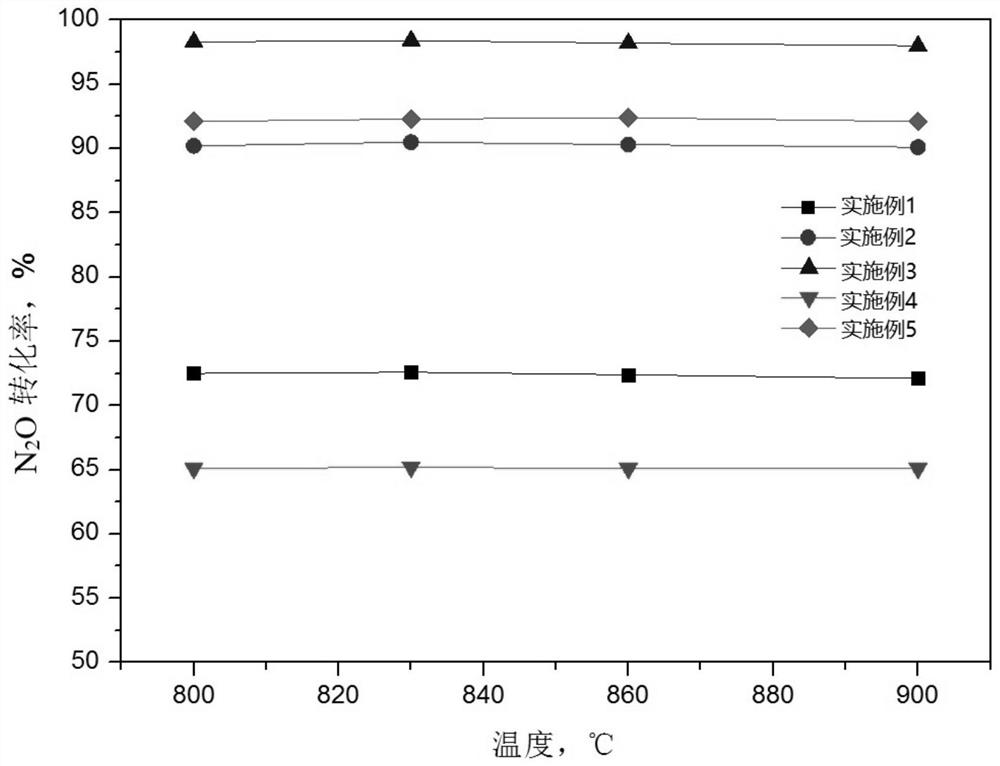
Ferro-cobalt bimetallic catalyst for catalyzing nitrous oxide (N2O) decomposition
InactiveCN102380410ANo significant decline in activityImprove stabilityNitrous oxide captureMolecular sieve catalystsDecompositionCatalytic oxidation
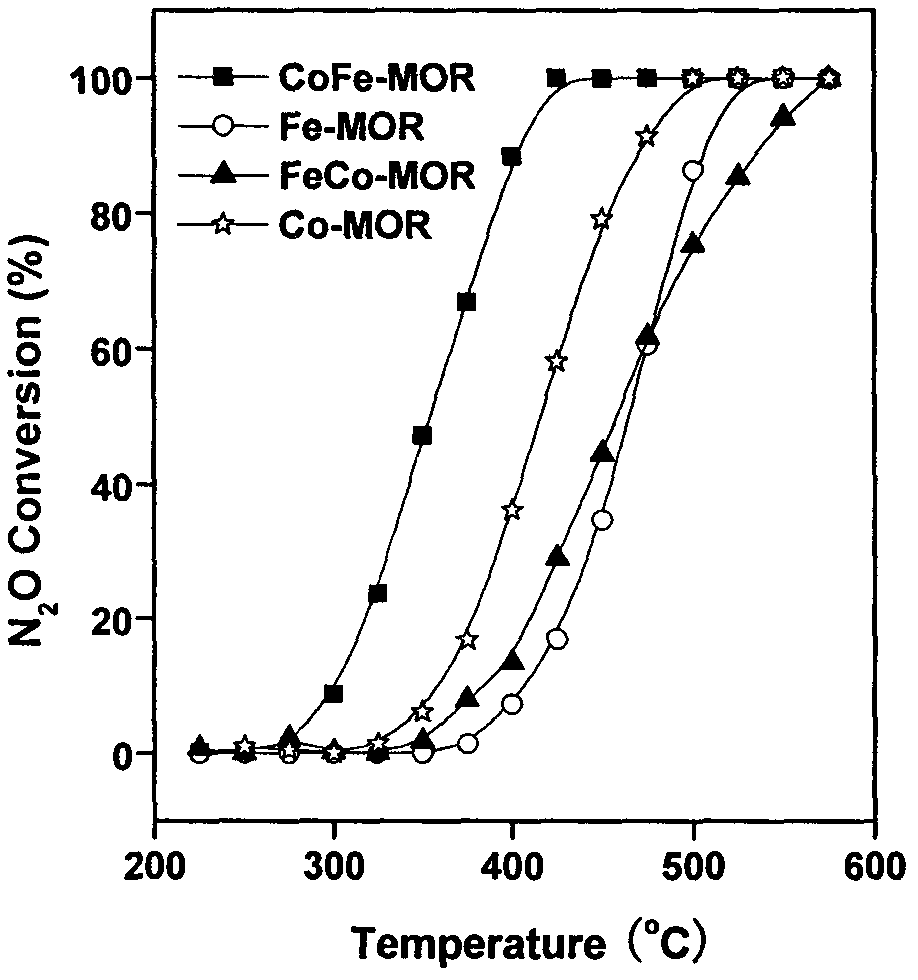
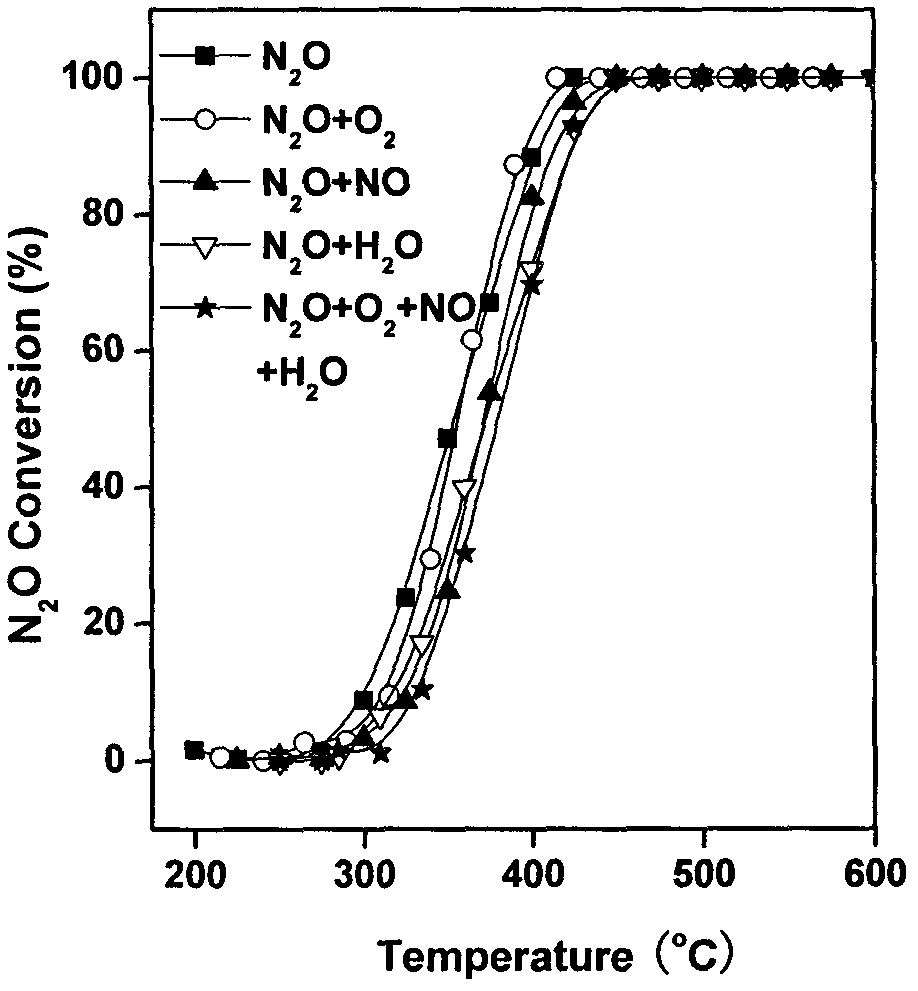
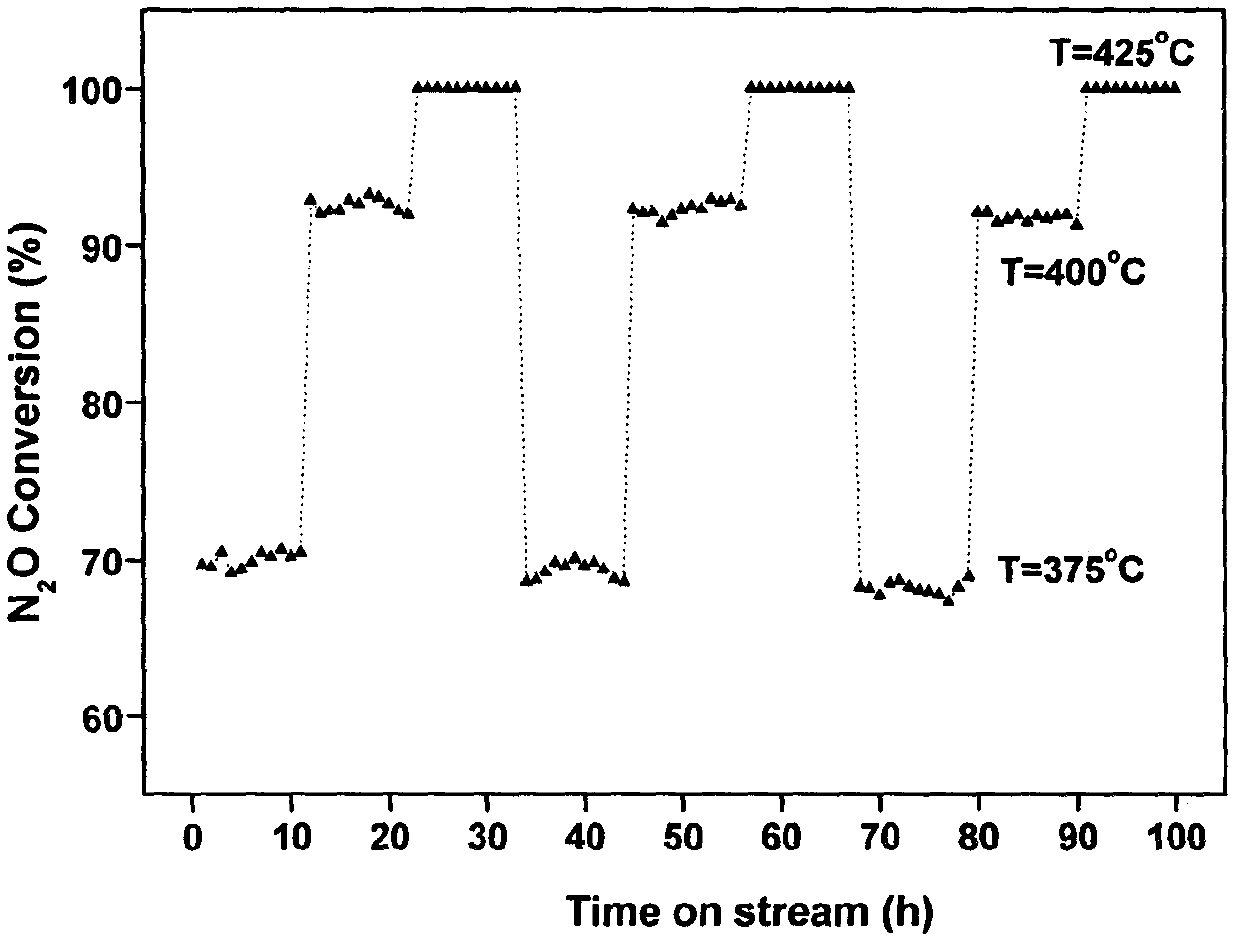
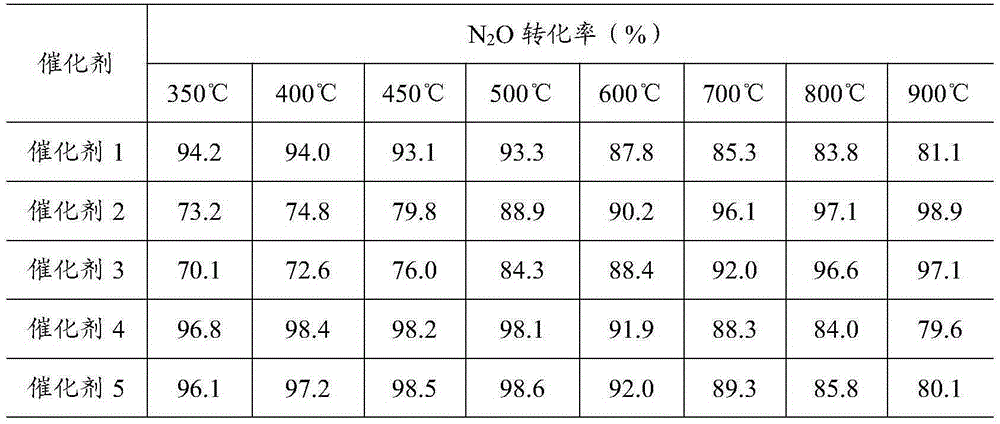
The invention provides a ferro-cobalt bimetallic load mordenite catalyst for catalyzing the nitrous oxide (N2O) decomposition and a preparation method. The catalyst can be used for decomposing most or all N2O in a pollution discharge system into N2 and O2 under the conditions that the space velocity is 30000h<-1>, the reaction temperature is 400 to 500 DEG C, the N2O concentration is 0.1 to 30 percent, and the H2O is 2 to 10 percent. In addition, the catalyst has high stability, and the activity of the catalyst is not decayed after 100h of the continuous nitric plant tail gas emission simulation. The catalyst has the advantages that the preparation process is simple, the cost is low, the catalyst is used for the catalytic N2O decomposition, the activity is high, and the stability is good. The technology has wide applicability, can be used for the N2O emission reduction process (such as processes of nitric acid production, adipate production and the like) of multiple industry sources and has wide application prospects.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Low-temperature activation efficient ammonia decomposition catalyst
PendingCN113181957AShorten crystallization timeReduce stackingFerrierite aluminosilicate zeoliteMolecular sieve catalystsMolecular sieveMetallic Nickel
The invention provides a low-temperature activation efficient ammonia decomposition catalyst and a preparation method thereof, and belongs to the field of ammonia decomposition catalysis. The catalyst is a nickel-containing FER molecular sieve; according to the molecular sieve, a nickel-containing carbon material is taken as a nickel source and a hard template agent, and metal nickel is introduced into the FER molecular sieve in situ; and the content of nickel in the nickel-containing molecular sieve is 0.1-5wt.% in terms of nickel element. the invention also provides a preparation method of the catalyst. The method specifically comprises the following steps of: adding a nickel-containing carbon material, a silicon source, an aluminum source, an organic template agent, inorganic base and a solvent into a reactor according to proportions; continuously stirring the materials to obtain uniform sol, aging the uniform sol; transferring the sol to a hydrothermal reaction kettle for crystallization; performing filtering, washing, drying and roasting on a product which is obtained after the reaction is finished; carrying out hydrogen transformation on an obtained molecular sieve; and finally roasting to obtain the nickel-containing FER molecular sieve. The catalyst disclosed by the invention has the advantages of rich microporous structure, short crystallization time and high catalyst activity and stability; the temperature required by ammonia decomposition is greatly reduced; and the catalyst has huge industrial application potential.
Owner:XIAMEN UNIV
Preparation method of supported medium and high temperature denitration catalyst
InactiveCN107754849ANot easy to pulverizeExtended service lifeMolecular sieve catalystsDispersed particle separationMolecular sieveHot Temperature
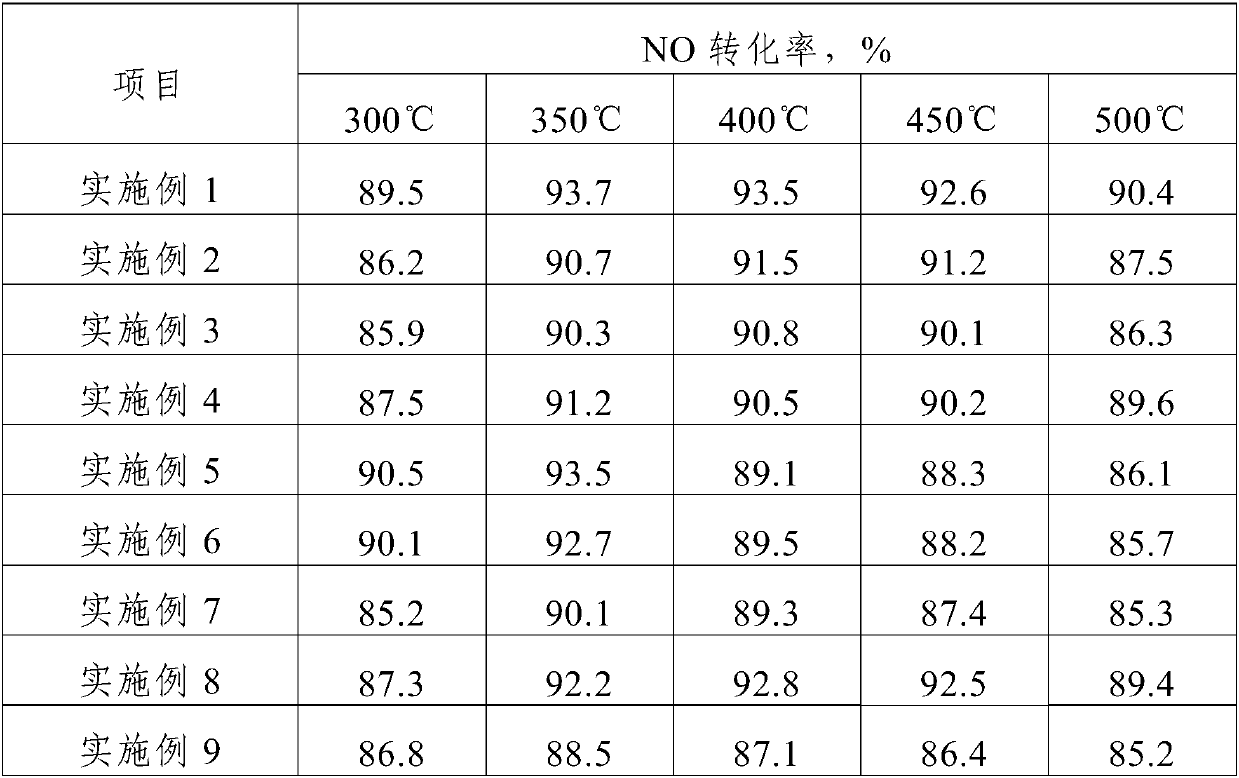
The invention discloses a preparation method of a supported medium and high temperature denitration catalyst. The method comprises the following steps: 1, dissolving a soluble active metal component precursor salt and a soluble auxiliary metal component precursor salt into deionized water to obtain a transparent impregnation solution; 2, impregnating a molecular sieve carrier for 6h-24h, filtering, drying at temperature of 80-120 DEG C for 12h-24h to obtain a catalyst precursor; 3, putting the catalyst precursor in a heat treatment furnace, heating to 300 DEG C-400 DEG C, calcining while introduction of air for 1h-3h, heating up to 600-800 DEG C, calcining for 2h-6h, and cooling to obtain the supported medium and high temperature catalyst. The supported medium and high temperature denitration catalyst prepared by the method has high strength, is not easy to pulverize, has long service life, and has low catalyst bulk density. The catalyst has a NO conversion rate of 85% or more at a suitable application temperature of 300 to 500 DEG C.
Owner:XIAN ORIGIN CHEM TECH
Titanium oxide supported sub-nano rhodium catalyst and preparation and application thereof
ActiveCN106861684AHigh activityEvenly dispersedDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsCatalytic decompositionAmmonium dinitramide
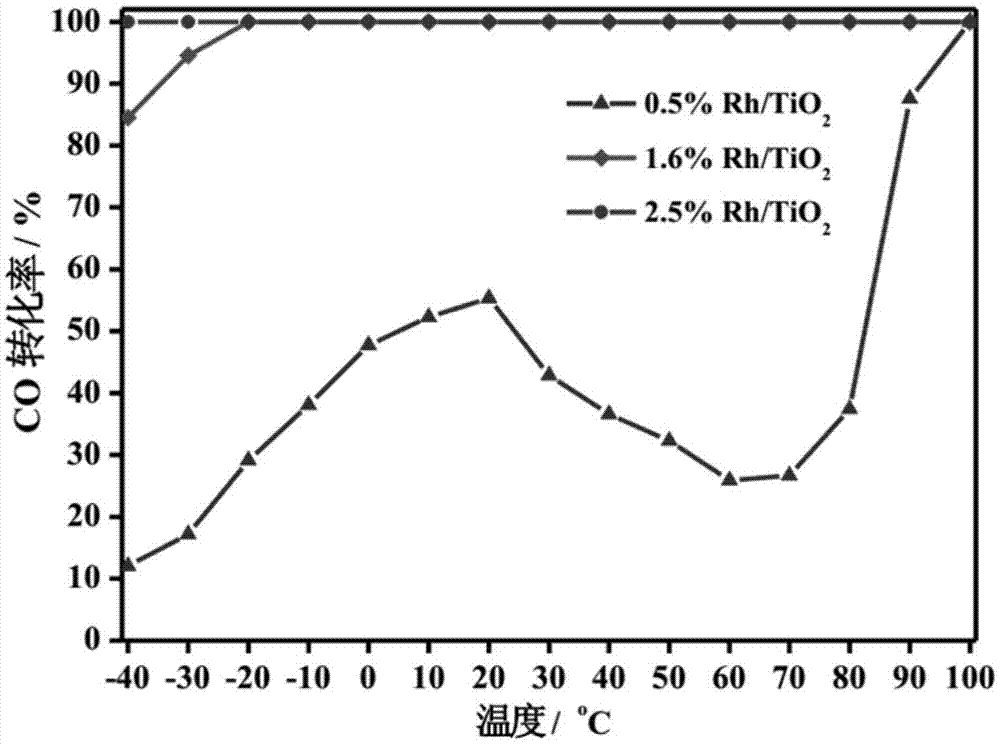
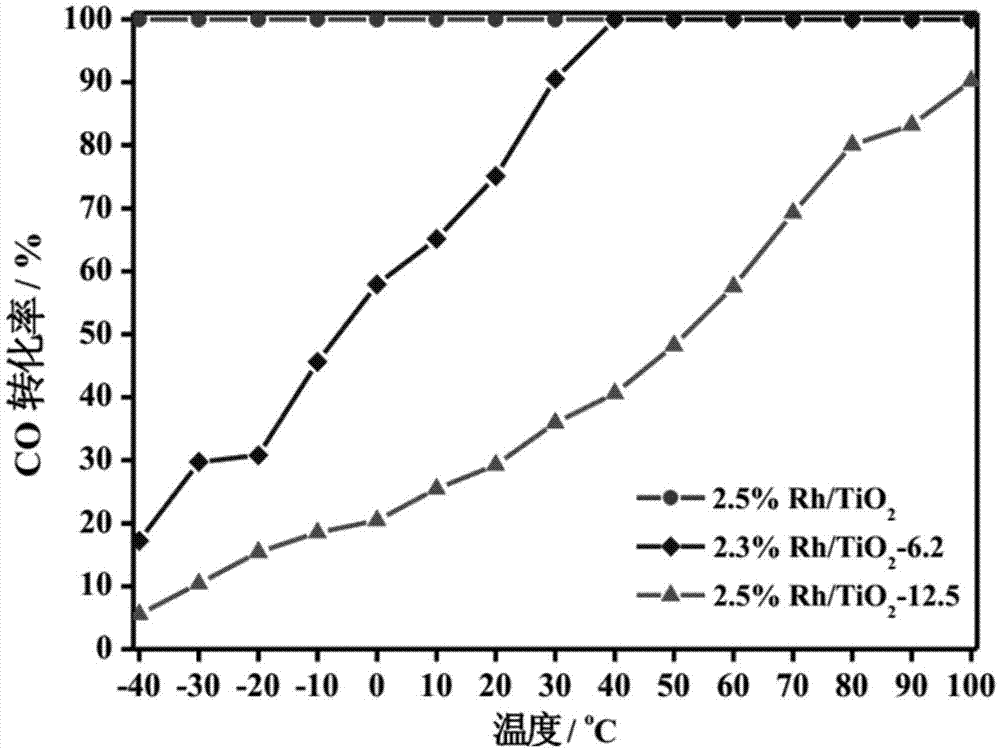
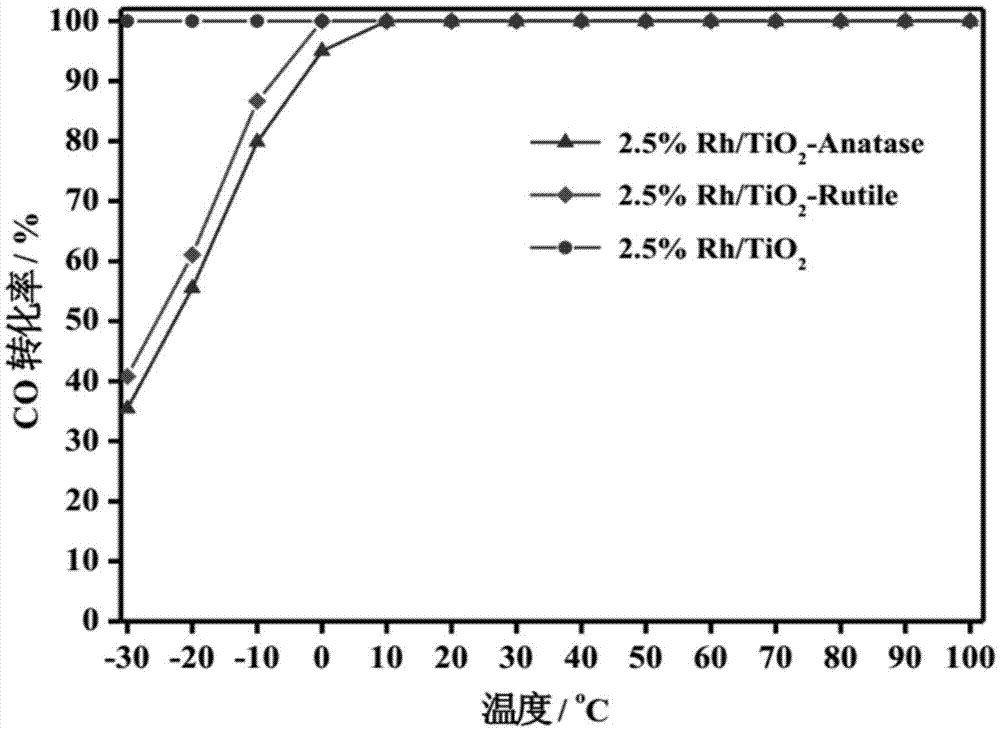
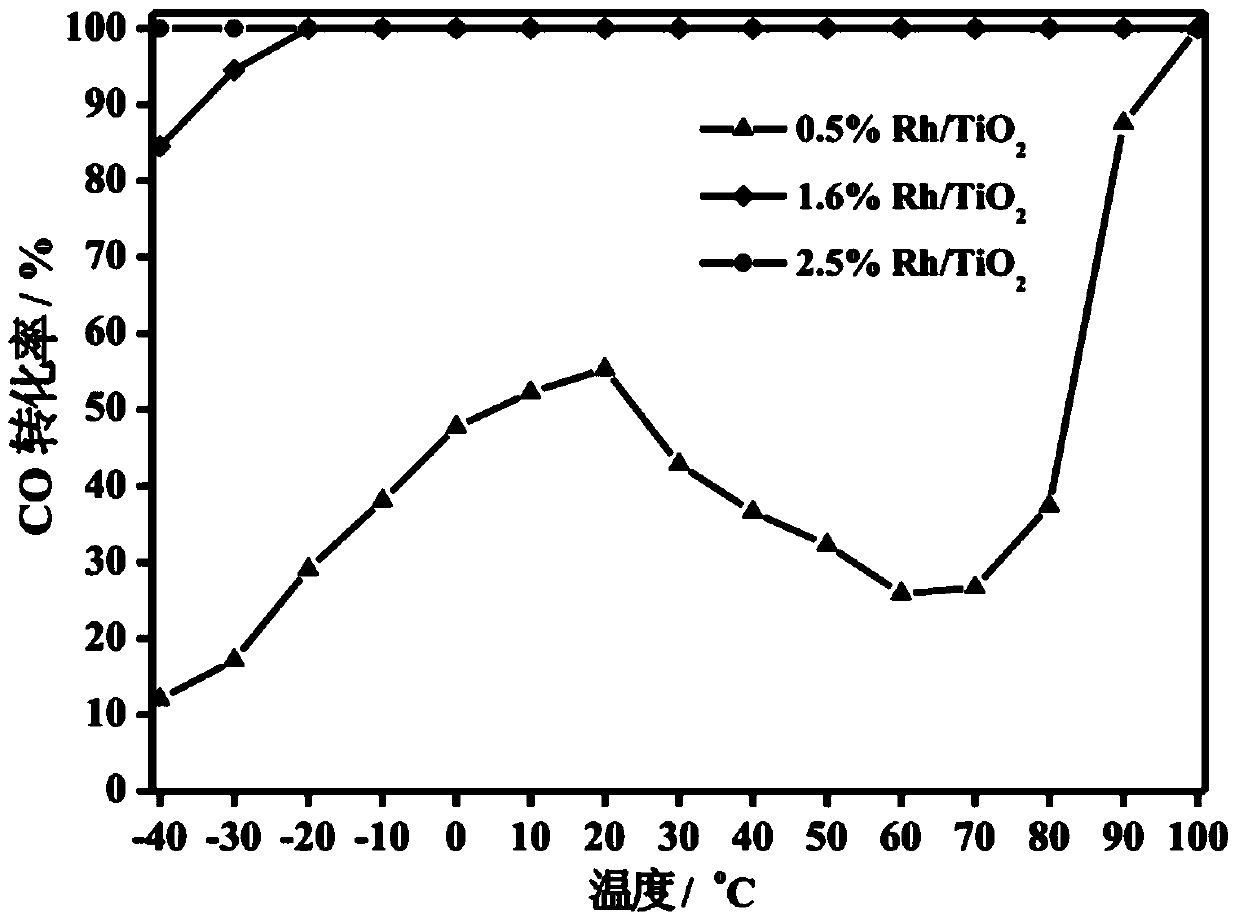
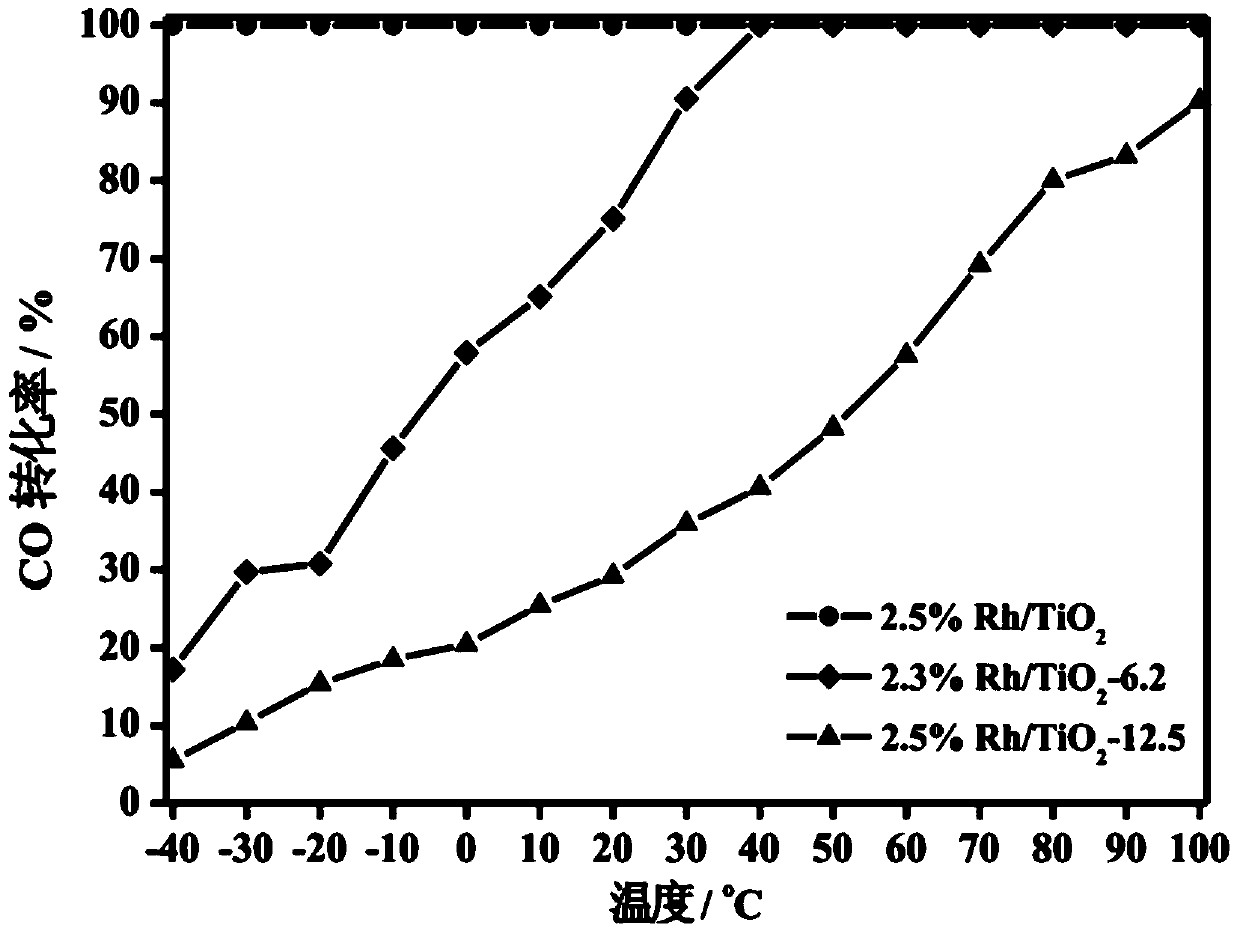
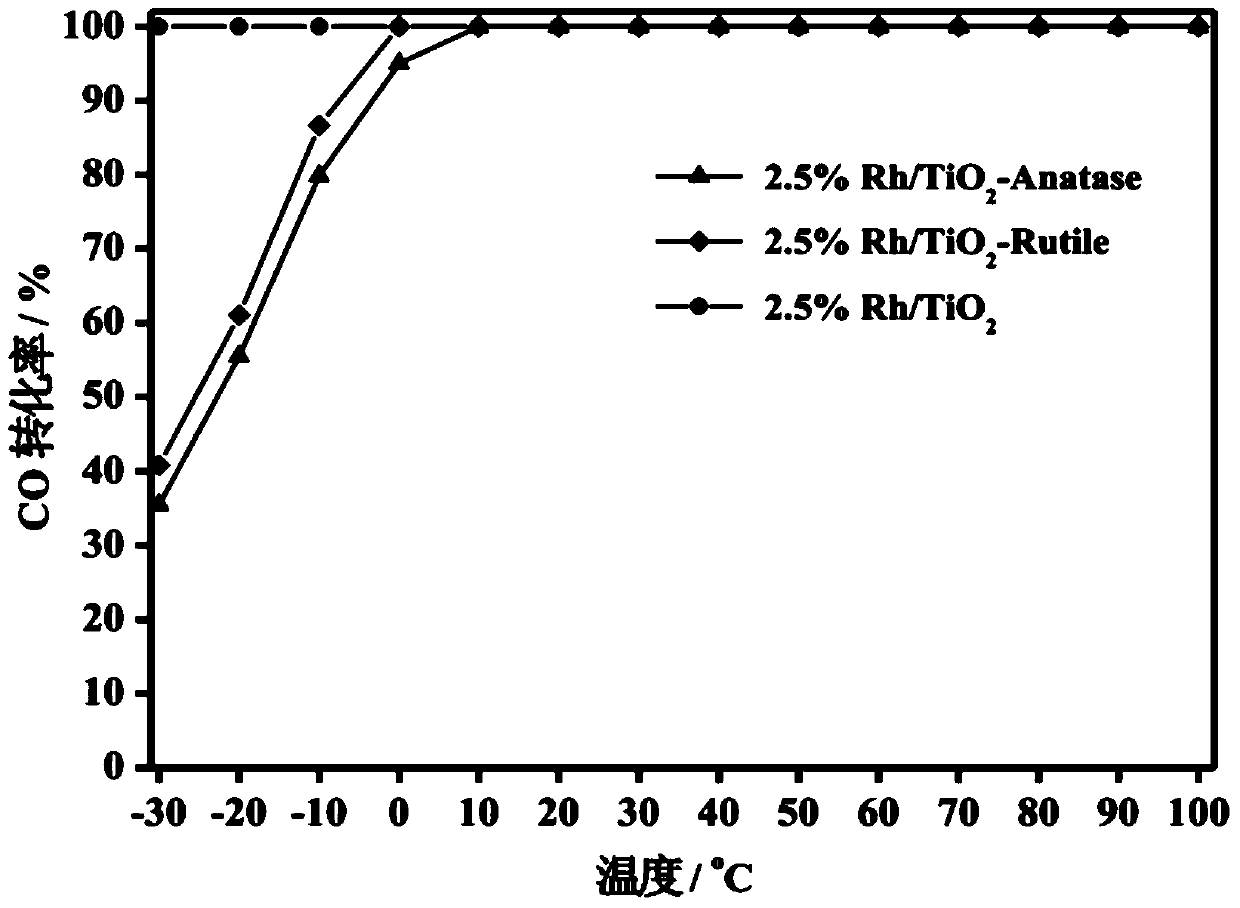
The invention relates to a titanium oxide supported sub-nano rhodium catalyst and an application thereof. Specifically, the rhodium content is 0.5-5% of the total mass of the catalyst, and is highly dispersed on a titanium oxide carrier in a sub-nano (0.5-1 nm) form. The rhodium dispersed on the catalyst carrier in the sub-nano form is used as an active center of a catalytic reaction, can be used for oxidation elimination of trace carbon monoxide (CO) in an ultra-low temperature environment and low temperature catalytic decomposition of liquid unit ammonium dinitramide (ADN) aerospace propellants.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Oyster mushroom cultivation method
InactiveCN105777408AFull of nutritionLoose textureSuperphosphatesCalcareous fertilisersCaladiumThiophanate-methyl
The invention discloses an oyster mushroom cultivation method. Oyster mushroom cultivation materials comprise, by mass, 47%-49% of cotton seed hulls, 19%-21% of Chinese silvergrass powder, 28%-32% of lotus seed hull powder, 0.8%-1.0% of calcium superphosphate, 0.9%-1.1% of gypsum powder and 0.1% of thiophanate methyl, wherein the mass fraction sum of all the components is 100%. When oyster mushrooms are cultivated, a mushroom shed is firstly set up, the raw materials are prepared and mixed, strain selecting, inoculating, mushroom shed disinfesting and sterilizing and spawn running are performed, and then mature oyster mushrooms can grow out. According to the oyster mushroom cultivation method, the idle land resources such as wasteland, waste mountains and forest open spaces around a house in countryside are reasonably utilized, waste utilization is achieved, pollution to the environment is reduced, the cultivation cost is low, and the method is extremely easy to apply and popularize in majority of peasant households, cooperatives and enterprises.
Owner:DAYE LONGFENGSHAN AGRI DEV GRP CO LTD
Catalyst for decomposing organic hazardous material and method for decomposing organic halides using the same
A catalyst for decomposing organic halides is characterized by comprising water-insoluble vanadyl sulfate or a composite catalyst comprising vanadyl sulfate, a specific oxide and a specific sulfate. This catalyst not only has excellent activity in decomposing organic halides, durability and resistance to SO2, but basically does not cause the oxidation of SO2 to SO3, and is also resistant to HCl, so it can be used for organic halides. The decomposition process has good efficiency and good durability, especially when dust and organic halides exist together, the gas to be treated contains SOx or HCl, or SOx or HCl is generated in the area to be treated.
Owner:MITSUI CHEM INC
Progressive slow piling steaming box
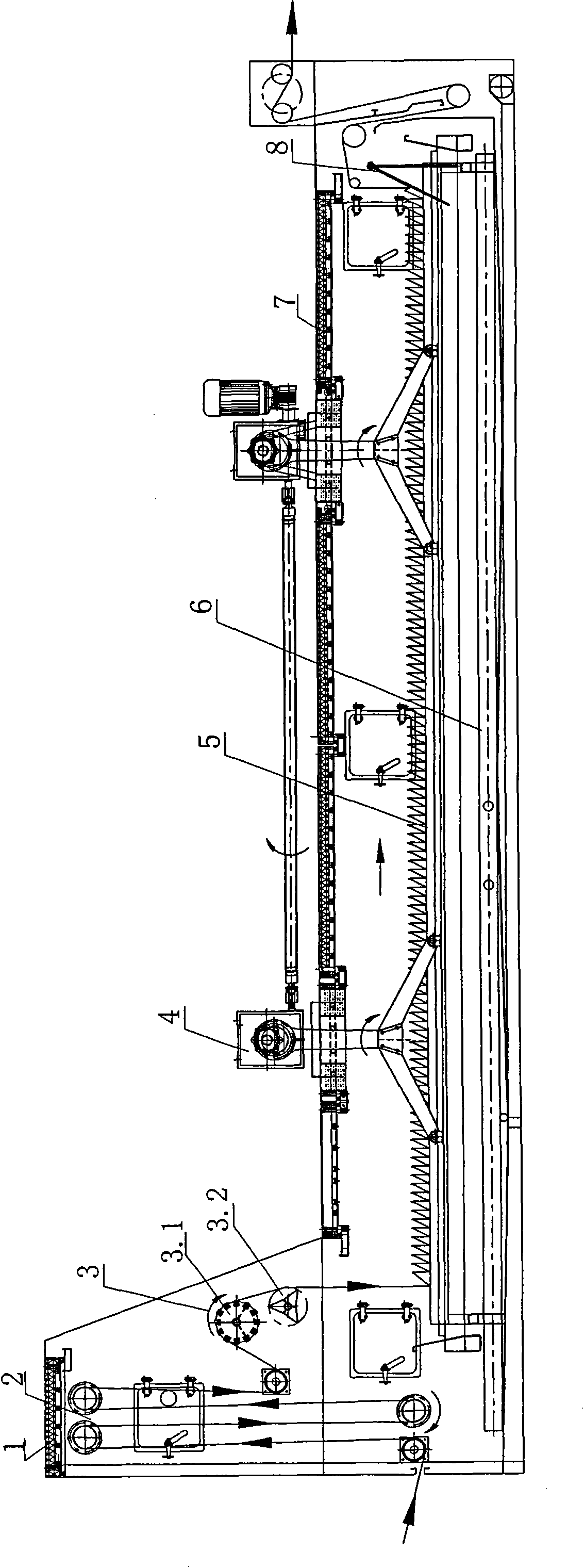
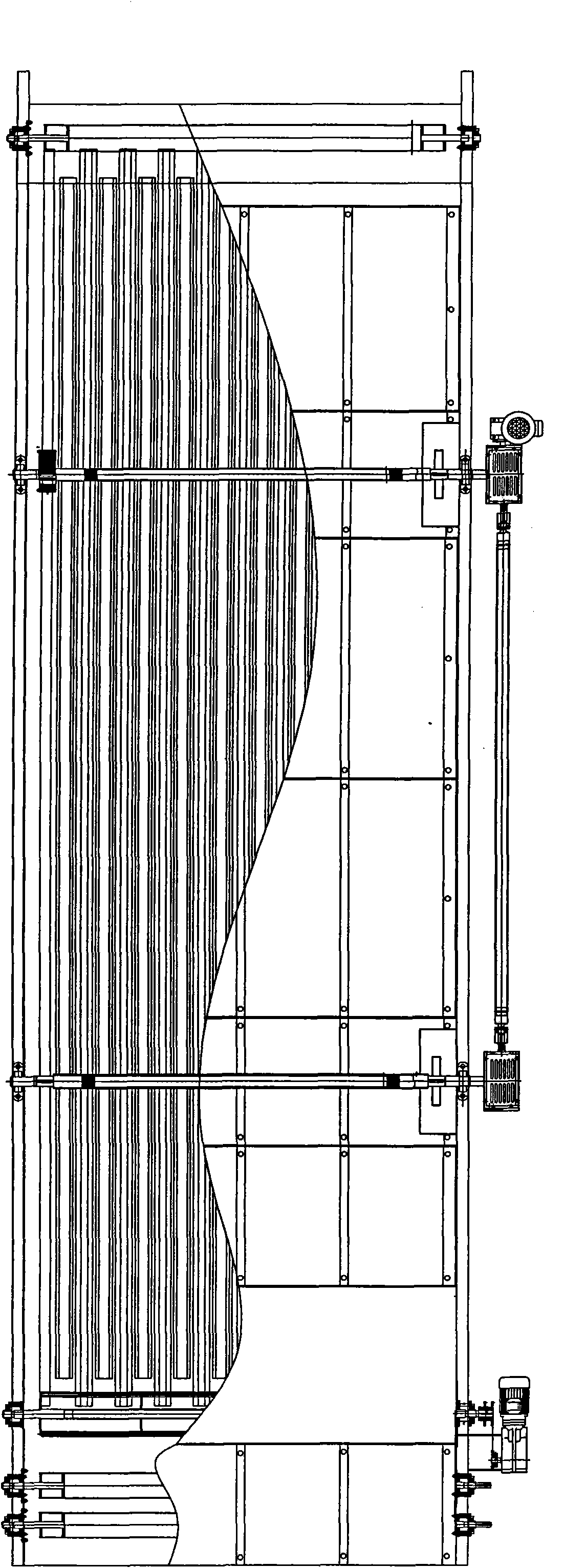
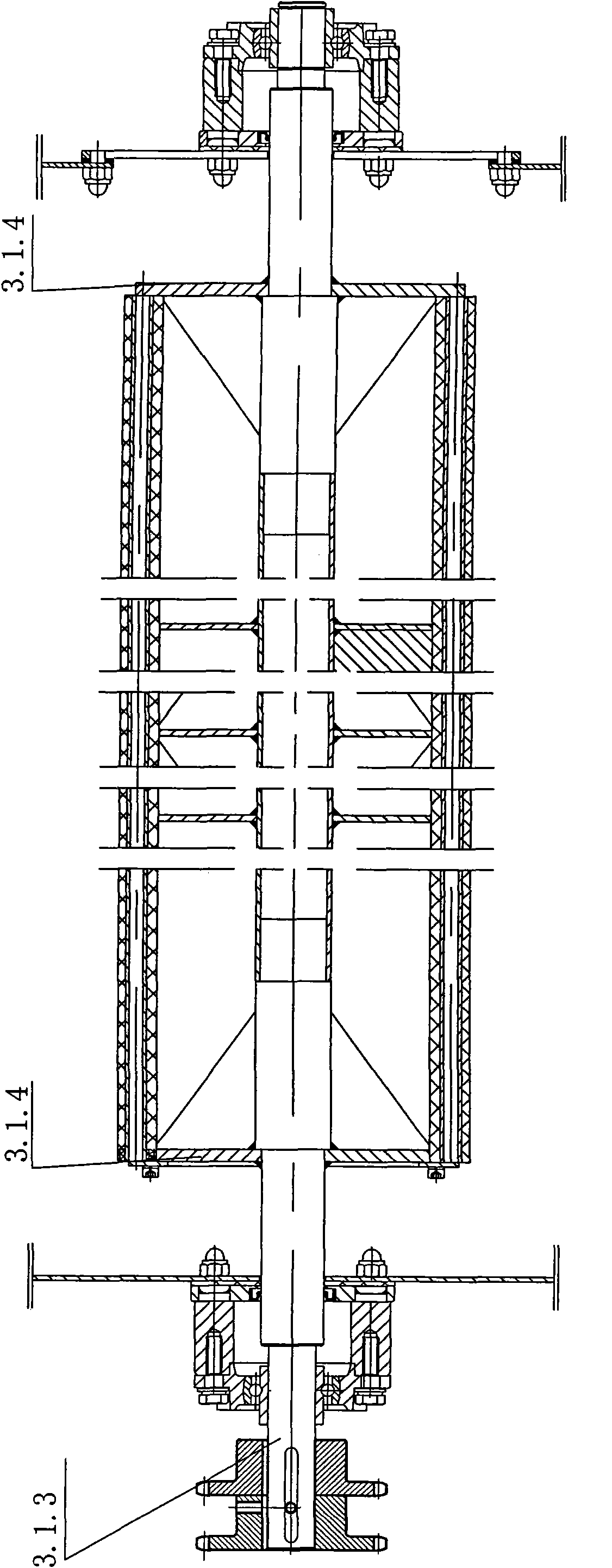
ActiveCN101892568AReduce stacking timeWon't scratchTextile treatment machine arrangementsTextile treatment carriersPolyesterDrive shaft
The invention relates to a progressive slow piling steaming box which is suitable for the steaming and the rinsing or the dying after the cold pad-batch pretreatment and the piling and the steaming before the printing of pure cotton and cotton-polyester blended fabrics. The steaming box comprises a box body (1), a progressive slow piling device (4) and a cloth supporting basket (5), wherein the progressive slow piling device (4) and the cloth supporting basket (5) are arranged in the box body (1); the progressive slow piling device (4) comprises a gear box motor (4.15), three transmission shafts (4.14), four rocker arm mechanisms and a cloth moving basket (4.16); a plurality of cloth supporting strips I (4.16.1) and a plurality of cloth supporting strips II (5.1) are convexly arranged on the cloth moving basket (4.16) and the cloth supporting basket (5) and distributed along the length direction of the box body; and the cloth supporting strips I (4.16.1) on the cloth moving basket (4.16) and the cloth supporting strips II (5.1) on the cloth supporting basket (5) are mutually spaced. The steaming box can ensure that the surfaces of fabrics cannot be scratched to have grinding defects and can not be scalded and has uniform and thorough steaming effect.
Owner:江阴福汇纺织有限公司
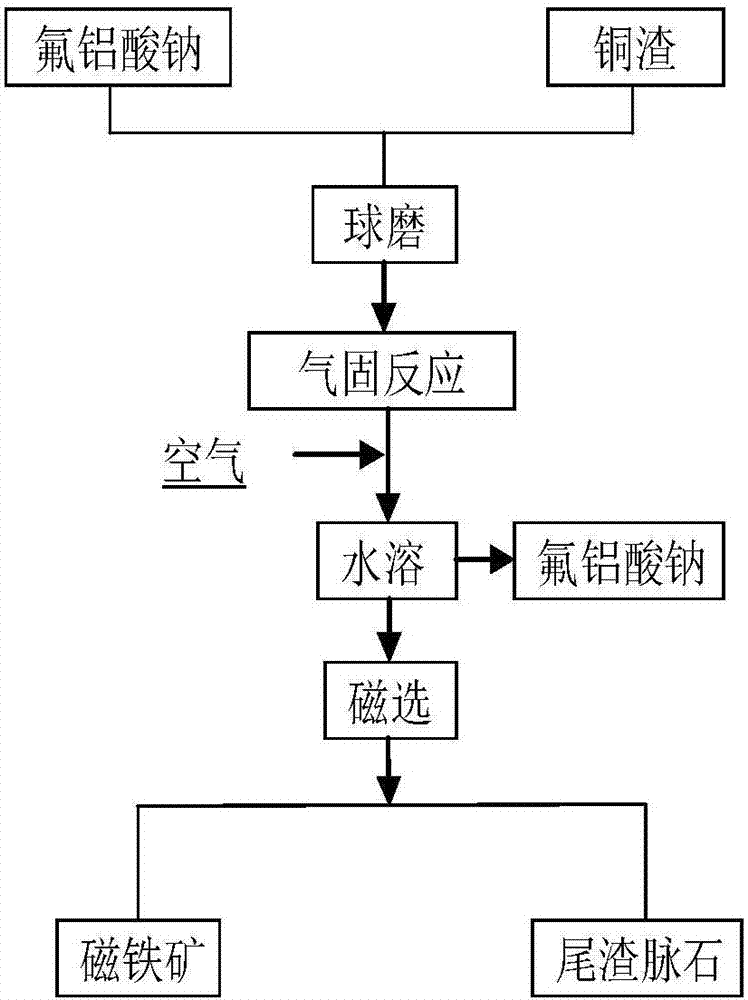
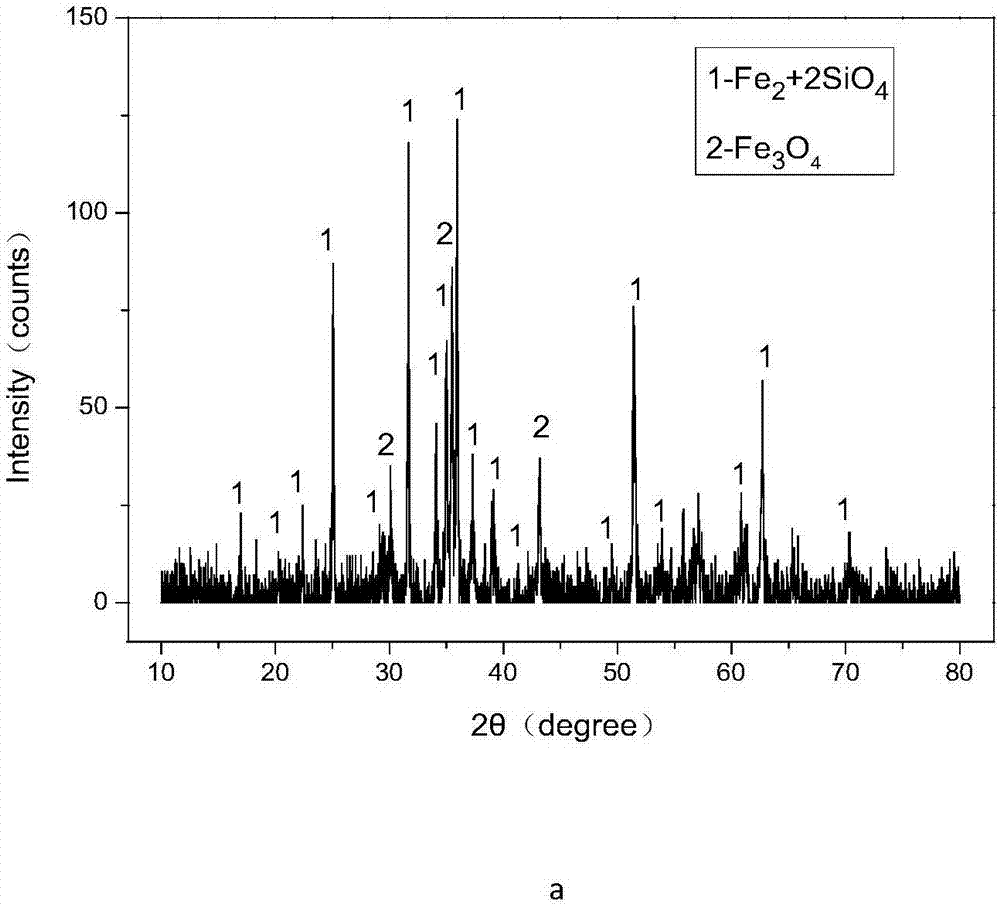
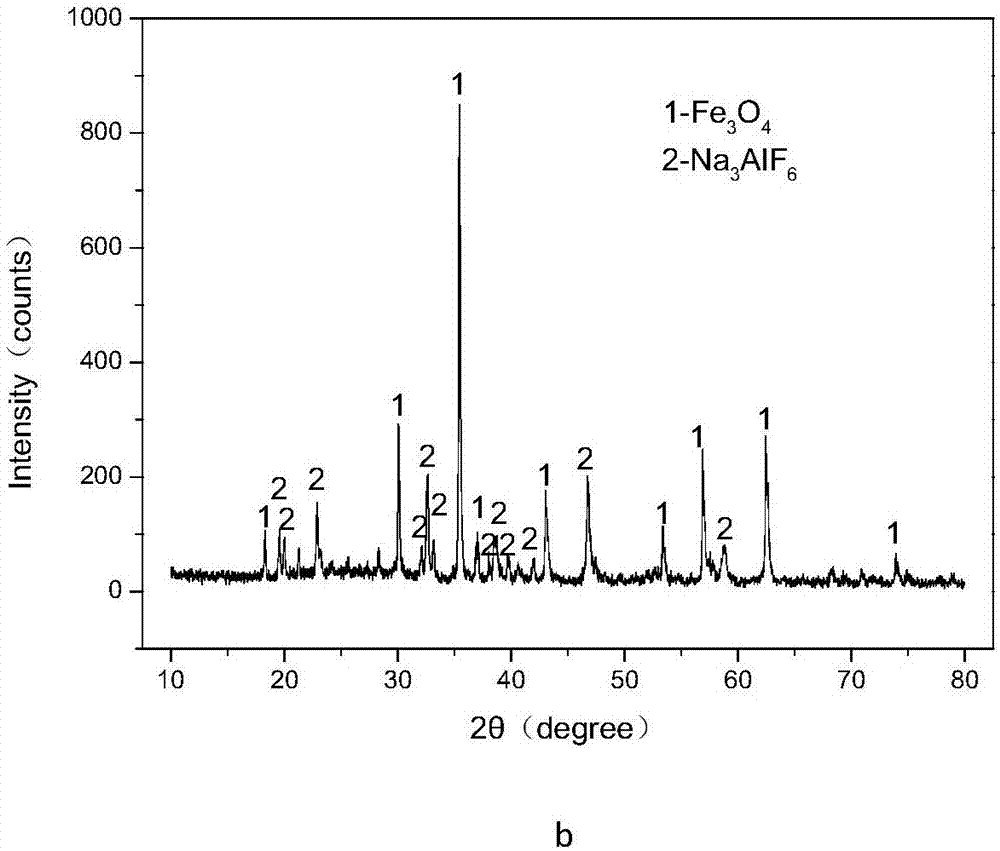
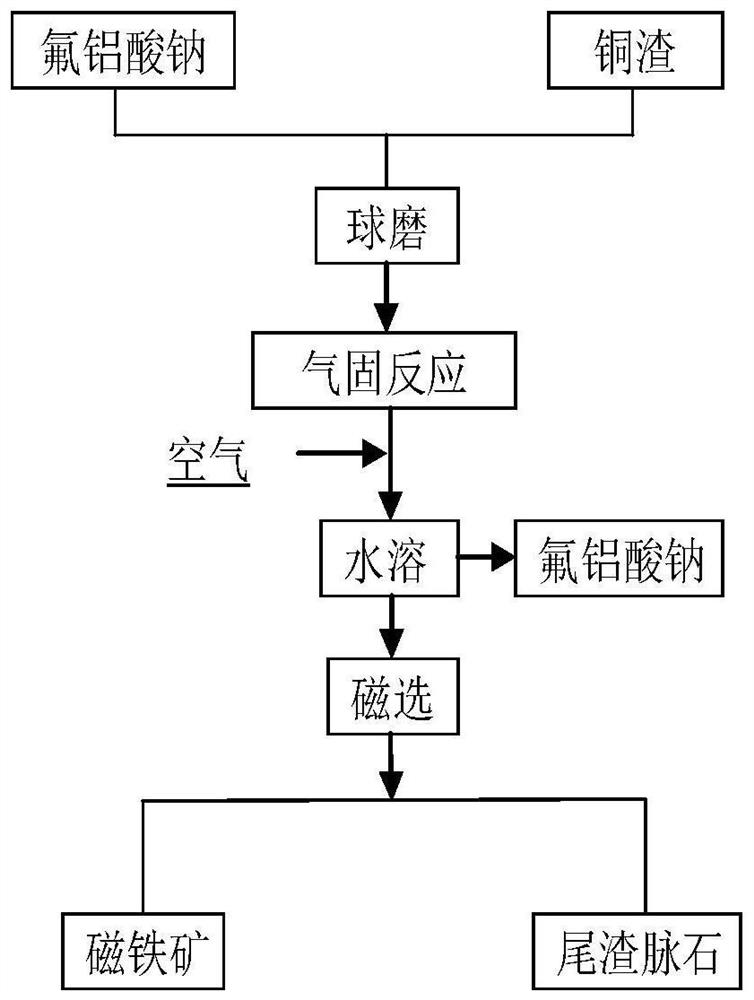
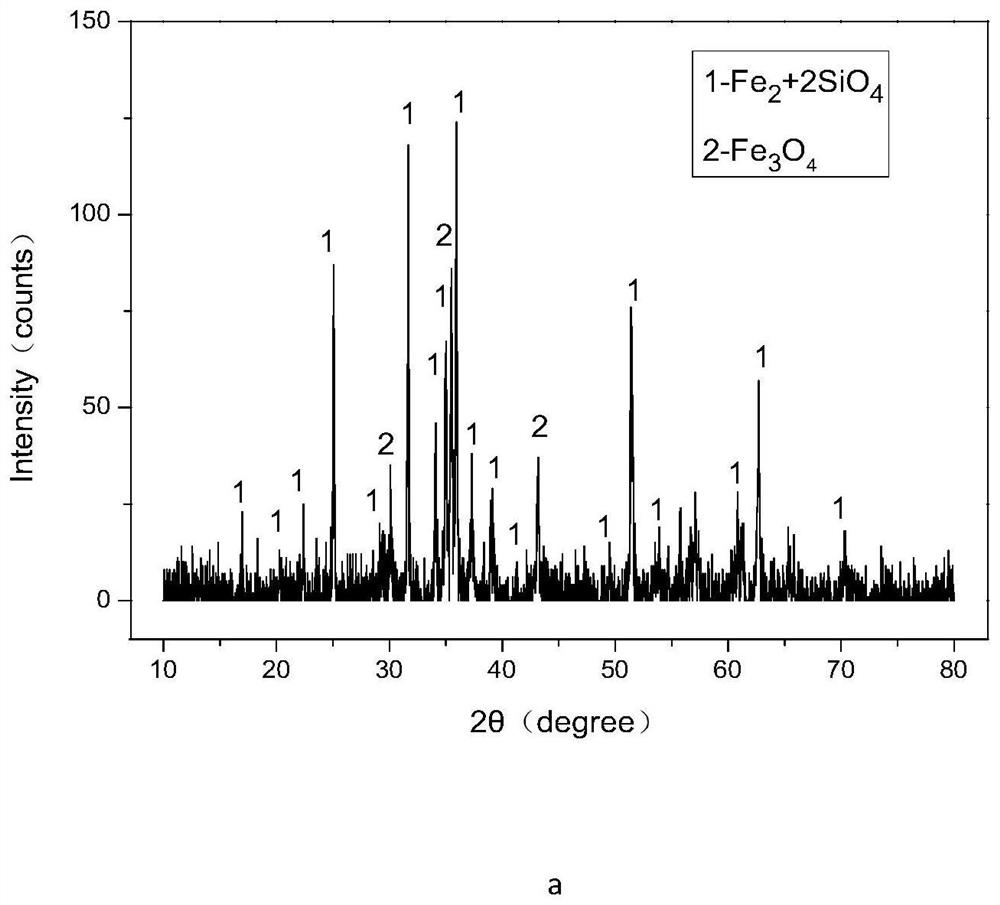
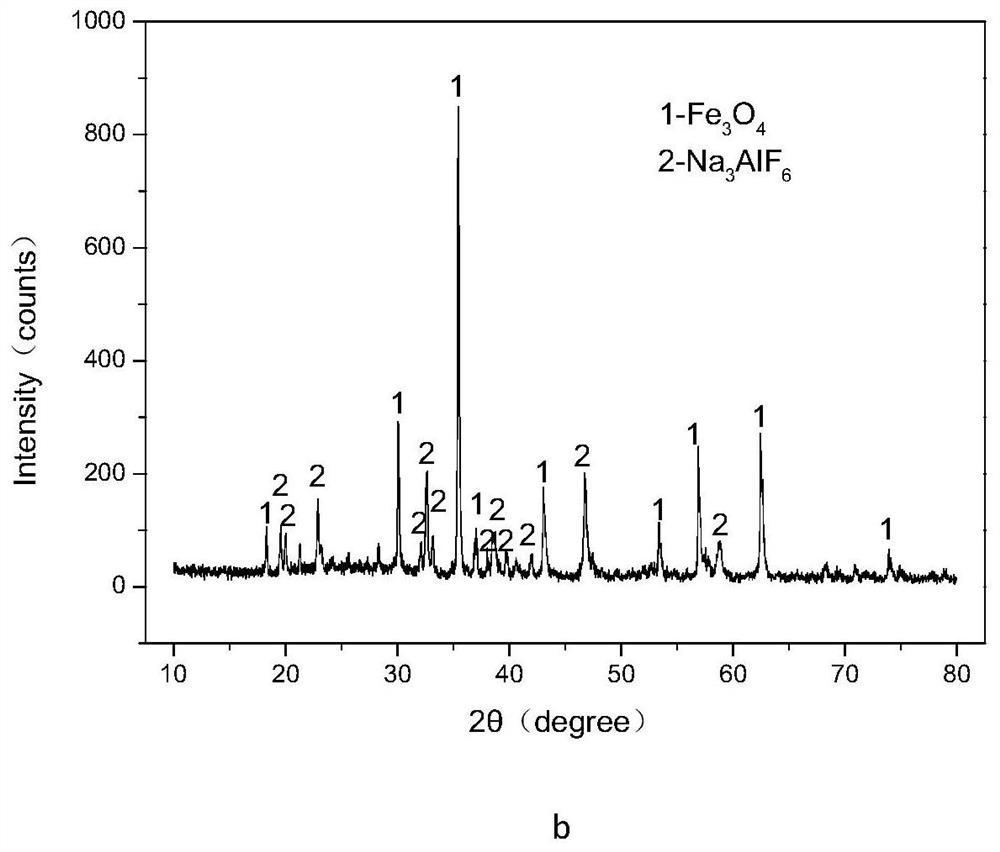
Method for efficiently decomposing and recycling valuable metal in copper slag
ActiveCN107955878ARealize magnetic separation recoveryLower decomposition temperatureMagnetic separationAir atmosphereMagnetite
The invention discloses a method for efficiently decomposing and recycling valuable metal in copper slag. According to the method, after the copper slag and cryolite are mixed and ball-milled, the mixture is arranged in an air atmosphere for calcination; calcination products are crushed and undergo magnetic separation, and then magnetite is obtained. By the adoption of the method for efficiently decomposing and recycling the valuable metal in the copper slag, iron resources incapable of being reused in the copper slag can be directionally regulated and controlled to be efficiently converted into the magnetite under mild conditions, reduction and reclamation of the copper slag are achieved, the copper slag tail end single-pass treatment problem, the national high-grade iron ore resource starvation problem, and the environmental pollution problem are solved, and a green sustainable development road is opened up for treatment of the copper slag.
Owner:CENT SOUTH UNIV
Composite catalyst for directly decomposing nitrogen oxide under rich oxygen condition
InactiveCN1473653ASimple preparation processRaw materials are easy to getMolecular sieve catalystsOrganic-compounds/hydrides/coordination-complexes catalystsDecompositionHigh activity
The present invention belongs to the field of pollution controlling technology, relates to catalyst for direct decomposition of nitrogen oxide, and is one kind of catalyst for direct decomposition ofnitrogen oxide under rich oxygen condition. The composite catalyst CuZSM-5 / MHn is prepared through mixing CuZSM-5 and MHn and roasting inside inert atmosphere at 500-800 deg.c for 2-3 hr. Inside the composite catalyst, MHn accounts for 5-15 wt%. The composite catalyst CuZSM-5 / MHn has high nitrogen oxide decomposing activity and high oxygen resisting activity. The composite catalyst has 20 % raised CuZSM-5 decomposition rate in the condition of oxygen content of 0-10 vol%, and unchanged high activity in the stability test of 10 hr.
Owner:DALIAN UNIV OF TECH
Catalyst for nitrous oxide decomposition and preparation method of catalyst
InactiveCN109622027ACoating stabilityShort preparation timeNitrous oxide captureGas treatmentDecompositionIon exchange
The invention relates to a catalyst for nitrous oxide decomposition and a preparation method of the catalyst. The preparation method comprises the following steps that 1) a silicon source, an aluminumsource and an organic template are added into water and adjusted to be alkaline to prepare a molecular sieve mother liquor; 2) a porous ceramic carrier is immersed in the molecular sieve mother liquor for crystallization; 3) metal ion exchange is performed on the crystallized porous ceramic carrier in step 2) to obtain a porous ceramic carrier containing a metal ion / molecular sieve; high-temperature calcination is carried out to obtain the catalyst. The molecular sieve in the preparation method is directly synthesized in situ on the porous ceramic carrier, a coating is firmly combined with the carrier, the catalyst is used for catalyzing N2O after the exchange with active components is performed, the degrading activity is high, and the water resistance is strong.
Owner:上海国瓷新材料技术有限公司
Catalyst for decomposing organic harmful substances and method for decomposing organic halides by use thereof
InactiveUS6858562B1Durability against HClLow costSulfate preparationLead oxidesPtru catalystVanadyl sulfate
A catalyst of a water insoluble vanadyl sulfate or a complex catalyst, in which a specific oxide and a specific sulfate are combined to the water insoluble vanadyl sulfate are excellent not only in their activity, durability and SO2 resistance, not only in substantially no oxidization of SO2 to SO3 as in HCl resistant. Therefore, using this catalyst, a decomposition treatment of an organic halide(s) can be carried out with high efficiency and good stability. In particular, a efficient decomposition treatment of an organic halides(s) can be carried out also in the cases that dust is coexist; the gas to be treated contains SOX or HCl; or they generate in the decomposition area.
Owner:MITSUI CHEM INC
Oyster mushroom cultivation method
InactiveCN106034737AComprehensive nutritionHigh biotransformation rateSuperphosphatesBioloigcal waste fertilisersCaladiumLand resources
The invention discloses an oyster mushroom cultivation method. An oyster mushroom culture material comprises, by mass, 45.8-47.8% of corn cob powder, 29-31% of wheat bran, 18-22% of miscanthus powder, 1.9-2.1% of calcium superphosphate, 0.9-1.1% of gypsum, 0.1% of urea, and 0.1% of carbendazim. A total of the mass percentages of the components is 100%. During a cultivation process, a mushroom shed is first built; the raw materials are prepared and mixed; a strain is selected and inoculated; the mushroom shed is subjected to pest killing and sterilization; and spawn running is allowed, such that mature oyster mushrooms can be obtained. According to the invention, free land resources such as wasteland, barren hill and forest open spaces among houses in the countryside are reasonably utilized, such that waste is utilized. With the method, environment pollution is reduced, and culture cost is low. The method can be easily popularized among farmers, cooperatives and enterprises.
Owner:DAYE LONGFENGSHAN AGRI DEV GRP CO LTD
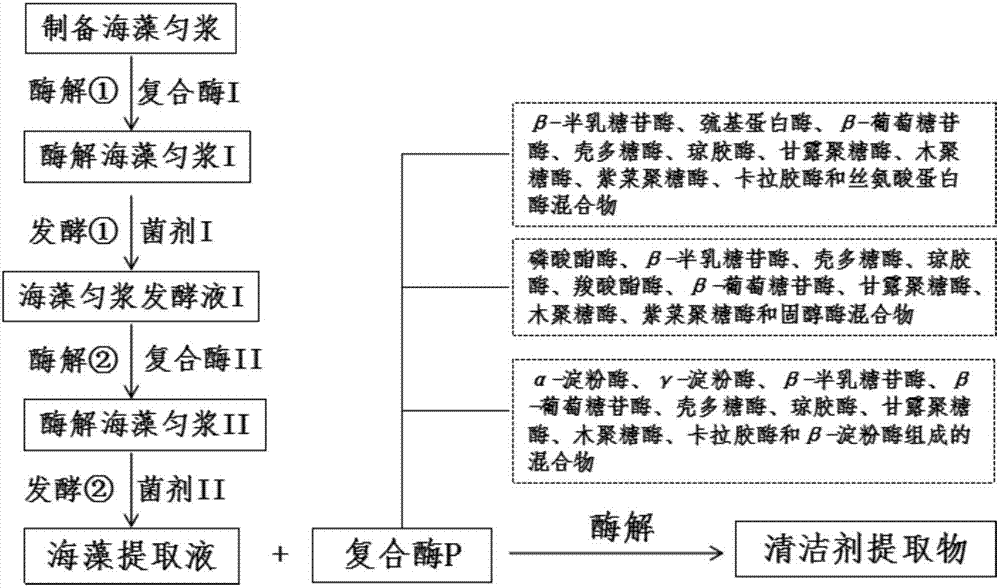
Cleaning agent and preparation method and application thereof
ActiveCN107201382AImprove extraction efficiencyAvoid pollutionCosmetic preparationsMicroorganismsActive componentNatural substance
The invention discloses a preparation method of a cleaning agent. The cleaning agent comprises a cleaning agent extract and water. The cleaning agent extract is prepared by steps: taking algae extract liquid, and adding complex enzyme P being 0.3-1.0% of the mass of the algae extract liquid to obtain algae-complex enzyme P mixed liquid; adjusting pH of the mixed liquid to 5-8, adjusting the temperature to 25-50 DEG C, and performing enzymolysis reaction for 24-72h to obtain the cleaning agent extract. By a biological fermentation process for production of cleaning antibacterial components, efficiency in extraction of active components in the extract is greatly improved; due to adoption of natural substances, environment pollution caused by the chemical cleaning agent is avoided; the method is simple and convenient, and a wide range in application is realized.
Owner:优艾斯(北京)科技有限公司 +1
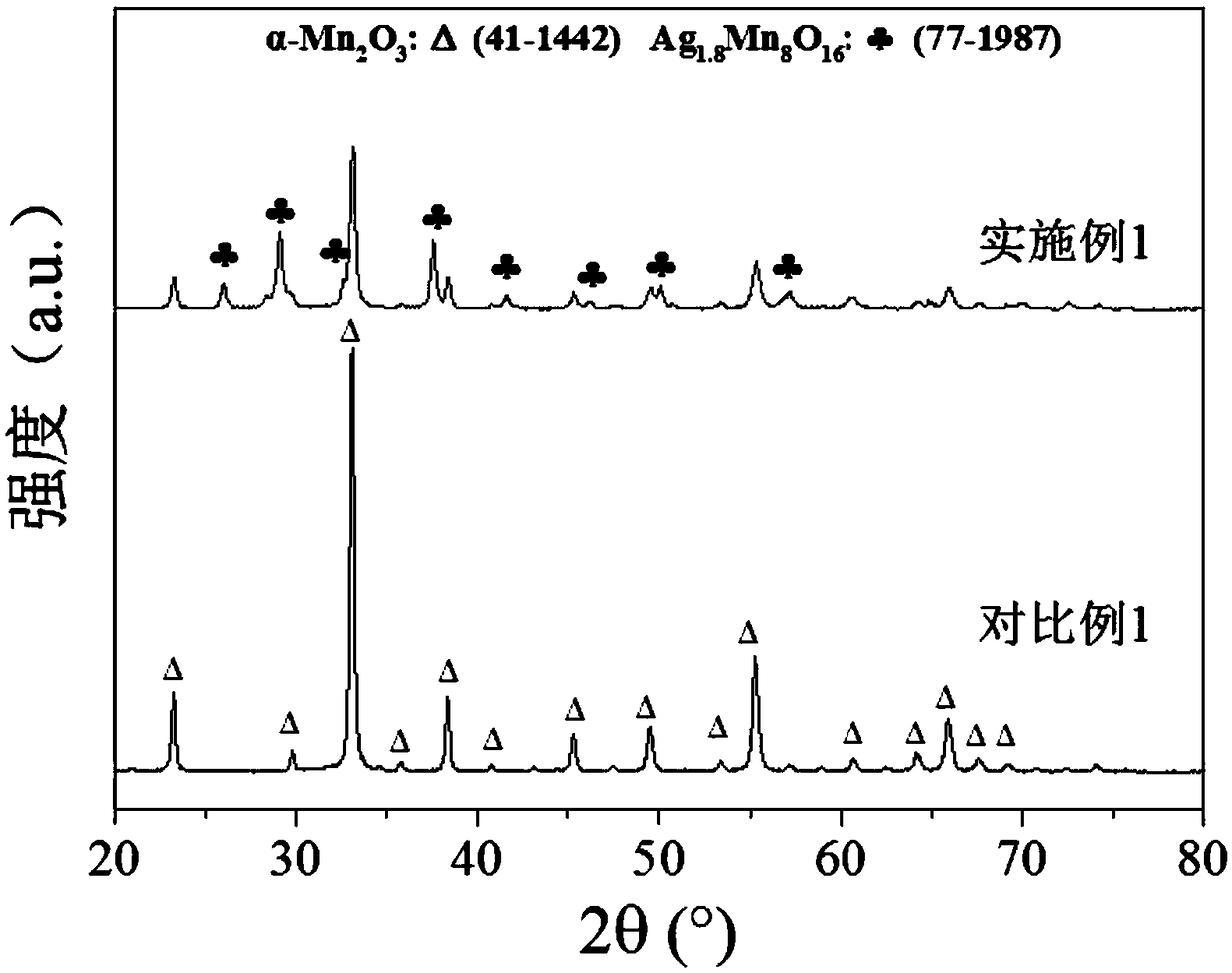
Silver-manganese oxide composite catalyst and preparation method and application thereof
ActiveCN109225218AQuick Migration PassImprove decomposition rateDispersed particle separationCatalyst activation/preparationDecompositionCrystal structure
The invention provides a silver-manganese oxide composite catalyst and a preparation method and application thereof. The preparation method comprises the following steps that an alkaline solution is added into a mixed solution of a manganese source and a silver source to react to generate solid precipitate; and the obtained solid precipitate is roasted to obtain the silver-manganese oxide composite catalyst. The silver-manganese oxide composite catalyst is prepared through a co-precipitation-roasting method, the catalyst forms a manganese-barium ore crystal structure, the electron transfer rate is high, the catalytic ozone decomposition activity is high, and when the relative humidity is about 70%, the ozone conversion rate can reach 80% or above in 6 h. The catalyst preparation method issimple and fast, the preparation process is high in controllability, adopted raw materials and the operating process are low in cost, and industrial production is facilitated.
Owner:中融创源科技发展有限公司
Polychlorinated biphenyl detoxifying complex composition and method for manufacturing same
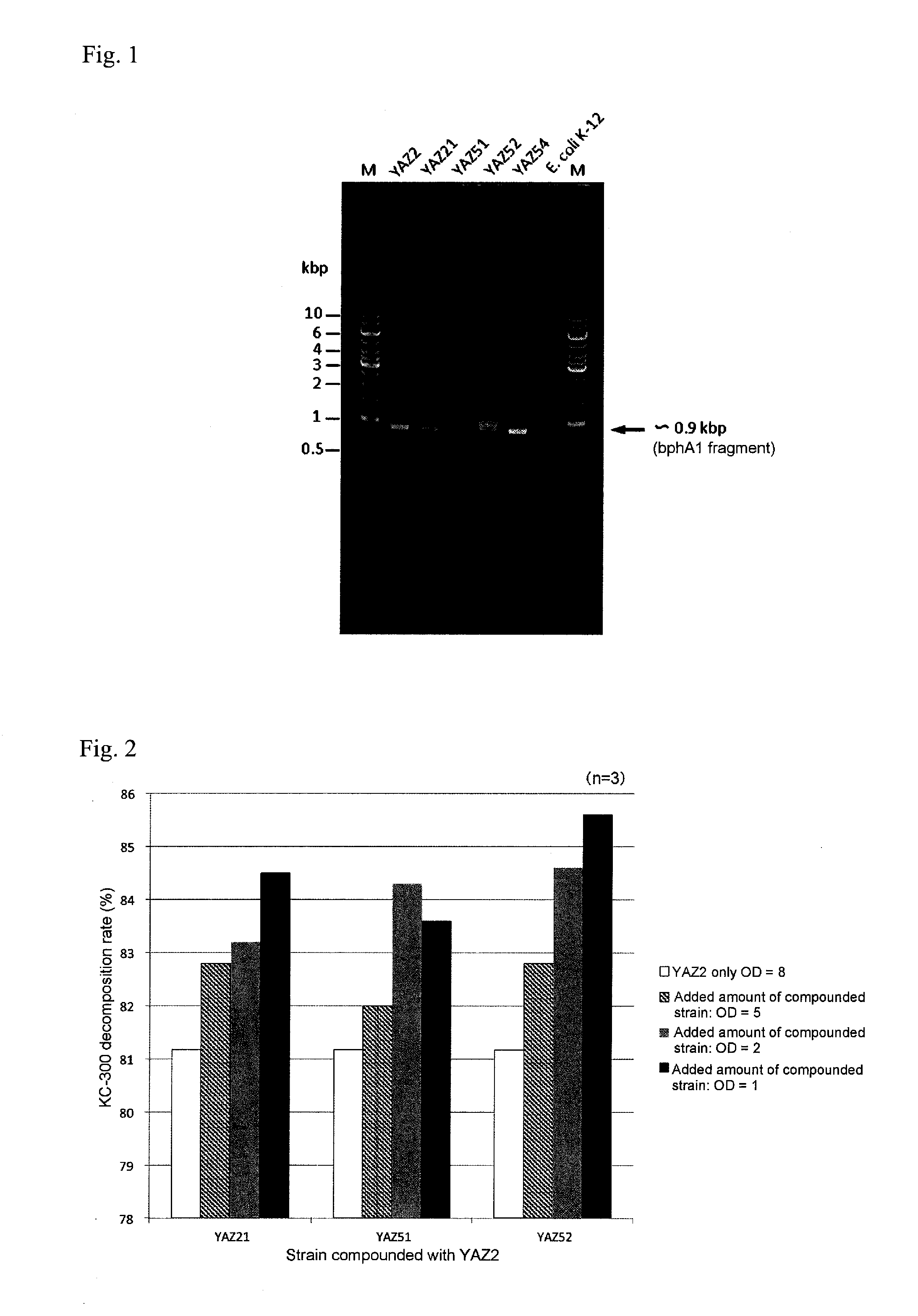
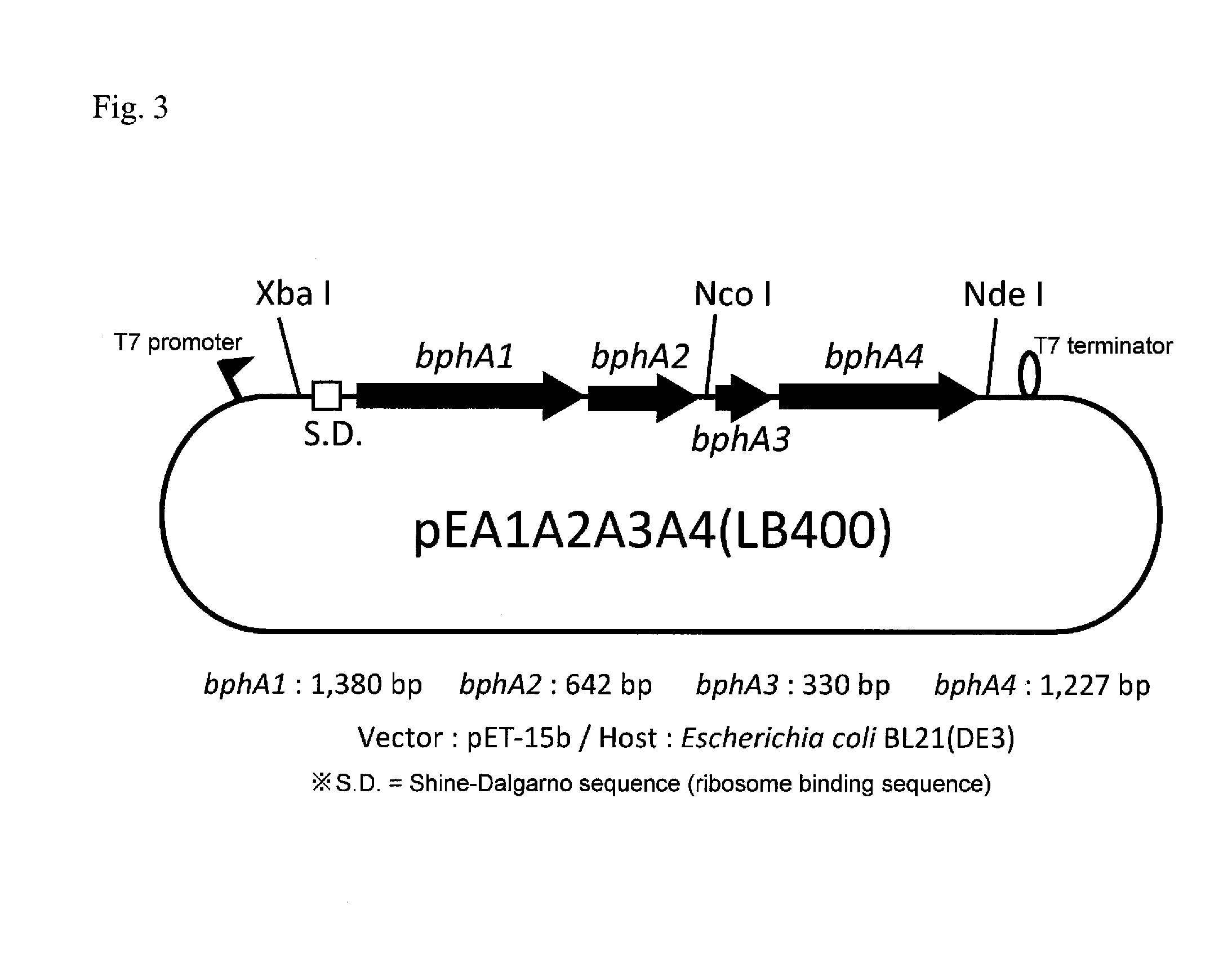
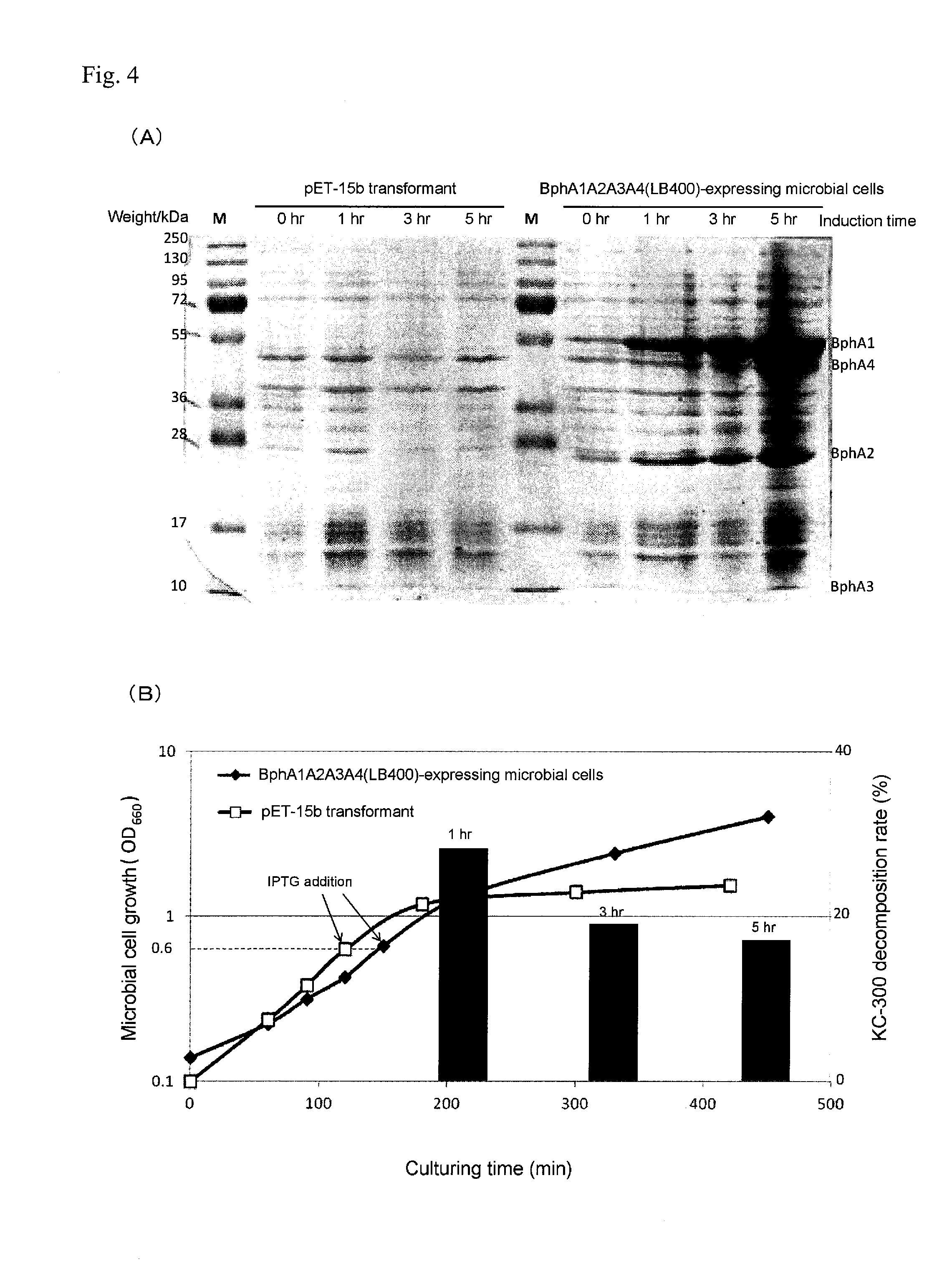
InactiveUS20160279456A1High PCB decomposition activityGood transportabilityBacteriaOxidoreductasesGenus AchromobacterMicroorganism
Provided is a polychlorinated biphenyl-decomposing composition obtained according to a method comprising respectively culturing at least one main microbial strain belonging to Comamonas species and having biphenyl dioxygenase, and at least one complementary microbial strain selected from the group consisting of Pseudomonas species, Achromobacter species, Rhodococcus species and Stenotrophomonas species and having biphenyl dioxygenase, and mixing at least two types of microbial cells recovered from each of the culture media. A composition containing these compounded microorganisms is useful for efficiently decomposing or detoxifying comparatively low concentrations of PCBs present in large amounts in waste products contaminated with polychlorinated biphenyls.
Owner:YAMAGATA UNIVERSITY
Preparation method of tea
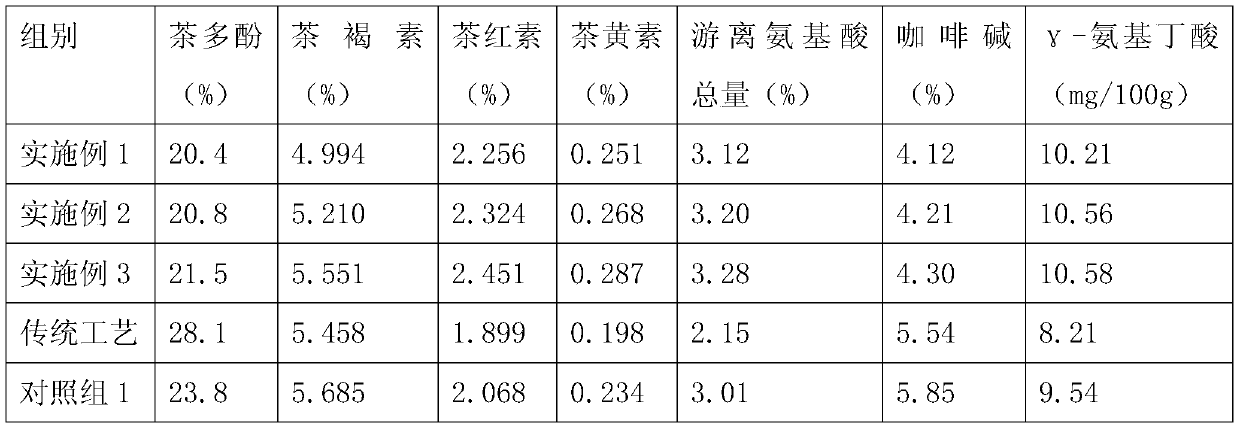
InactiveCN109924286APromote oxidationReduce the concentration of soup colorPre-extraction tea treatmentFermentationChemistry
The invention belongs to the technical field of tea processing and particularly relates to a preparation method of tea. The preparation method specifically comprises the following operation steps: (1)picking fresh leaves; (2) withering; (3) rolling: putting the withered tea leaves into a rolling machine for rolling, installing an extraction type ice cube box at the lower end of a rolling disc ofthe rolling machine, adding ice cubes into the extraction type ice cube box, wherein the height of the extraction type ice cube box is 15-20 cm, the height of the added ice blocks is 2 / 3 of the heightof the full extraction type ice block box every time, rolling for 20-25 minutes, and stopping rolling; (4) fermenting; (5) frying: after fermentation is completed, executing drying at 90-120 DEG C. The preparation method has the advantage that the quality of the tea can be effectively improved.
Owner:广西广茗投资有限公司
Catalyst for catalytic decomposition of nitrous oxide and preparation method of catalyst
ActiveCN105289654AEasy to makeEasy to scale up productionNitrous oxide captureDispersed particle separationCerium(IV) oxideWater vapor
The invention discloses a load type oxide catalyst for directly decomposing nitrous oxide. The catalyst is prepared from a carrier and active components, wherein the active components are cobalt, aluminum, zirconium and lanthanum, assistants are platinum, rhodium, palladium and ruthenium, and the carrier is ceric oxide. In addition, the invention also discloses a preparation method of the catalyst. The catalyst has better nitrous oxide direct-decomposing activity and high vapor resistance, has higher activity in a wider temperature range, is strong in stability and free of inactivation after a continuous 50h reaction, and has a conversion rate of more than 90 percent.
Owner:XIAN ORIGIN CHEM TECH
Catalyst for purification of exhaust gases, production process therefor, and process for purification of exhaust gases
InactiveUS20010031700A1High decomposition activityGood dispersionNitrogen compoundsHeterogenous catalyst chemical elementsVanadium oxideTitanium oxide
The present invention provides: a catalyst for purification of exhaust gases which catalyst is excellent as a denitrification catalyst which has still more excellent ability to remove nitrogen oxides and of which the ability to oxidize sulfur dioxide into sulfur trioxide is extremely suppressed and further as a catalyst which is favorable for efficiently removing organohalogen compounds, such as dioxins, from exhaust gases; a production process therefor; and a process for purification of exhaust gases. The catalyst for purification of exhaust gases comprises titanium oxide, molybdenum oxide, and vanadium oxide as catalytic components, wherein the titanium oxide and the molybdenum oxide are included in the catalyst in the form of: a binary closely mixed oxide which is beforehand prepared and includes titanium and molybdenum; and / or a trinary closely mixed oxide which is beforehand prepared and includes titanium, silicon, and molybdenum.
Owner:NIPPON SHOKUBAI CO LTD
Ammonia decomposition catalyst
InactiveCN102333590ALarge specific surface areaPrevent agglutinationMaterial nanotechnologyCatalyst activation/preparationAlkaline earth metalDecomposition
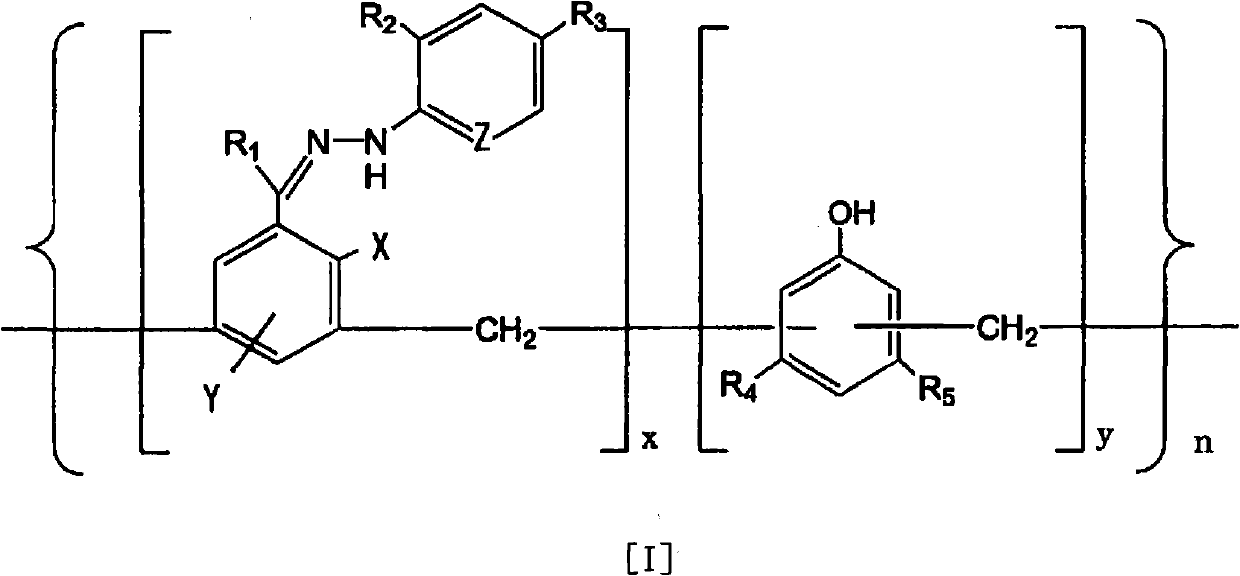
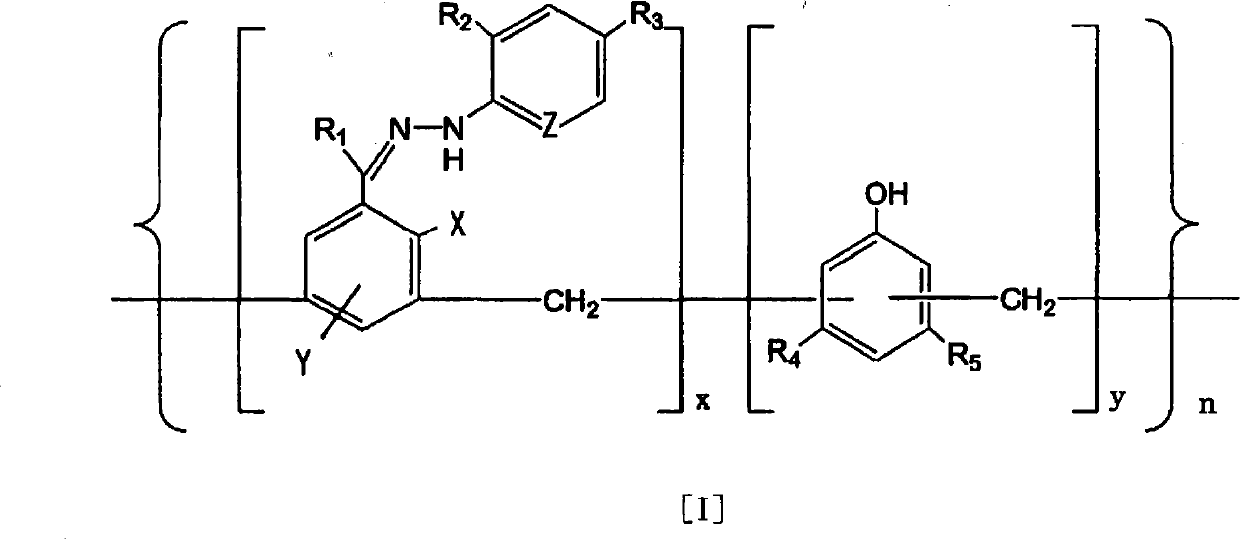
Disclosed is an ammonia decomposition catalyst which is obtained by subjecting a complex to a heat treatment at a temperature of 360-900 DEG C in a reducing atmosphere, said complex being obtained by coordinating a transition metal to a polymer that is represented by formula (I) and has a molecular weight of 1,000-500,000, and adding activated carbon or a carbon nanotube to the polymer. In the case when a carbon nanotube is added, an alkali metal compound or an alkaline earth metal compound is added to the heat-treated complex. In formula (I), R1 represents H or a hydrocarbon group having 1-10 carbon atoms; R2 and R3 each represents H, a halogen, a nitro group, an acyl group, an ester group, a carboxyl group, a formyl group, a nitrile group, a sulfone group, an aryl group or an alkyl group having 1-15 carbon atoms; X and Y each represents H or OH; Z represents CH or N; R4 and R5 each represents H, OH, an ether group, an amino group, an aryl group or an alkyl group having 1-15 carbon atoms; x represents a real number between 1 and 2; y represents a real number between 1 and 3; and n represents a real number between 2 and 120. The ammonia decomposition catalyst is capable of increasing the amount of transition metal loaded thereon without lowering the dispersion of the transition metal, and is capable of reducing the use amount of catalyst necessary for obtaining desired activity.
Owner:HITACHI ZOSEN CORP +2
A kind of titanium oxide supported subnano rhodium catalyst and its preparation and application
ActiveCN106861684BHigh activityEvenly dispersedDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsCatalytic decompositionAmmonium dinitramide
The invention relates to a subnano rhodium catalyst supported by titanium oxide and its application. Specifically, the rhodium content is 0.5-5% of the total mass of the catalyst, and it is highly dispersed on the titanium oxide carrier in the form of sub-nanometer (0.5-1 nm). The subnano-dispersed rhodium on the catalyst carrier of the invention is used as the active center of the catalytic reaction, and can be used for the oxidation elimination of trace carbon monoxide (CO) in an ultra-low temperature environment and the low-temperature catalytic decomposition of liquid unit ammonium dinitramide (ADN) aerospace propellant.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Mutants of penicillin G acylase from Achromobacter sp. CCM 4824 and uses thereof
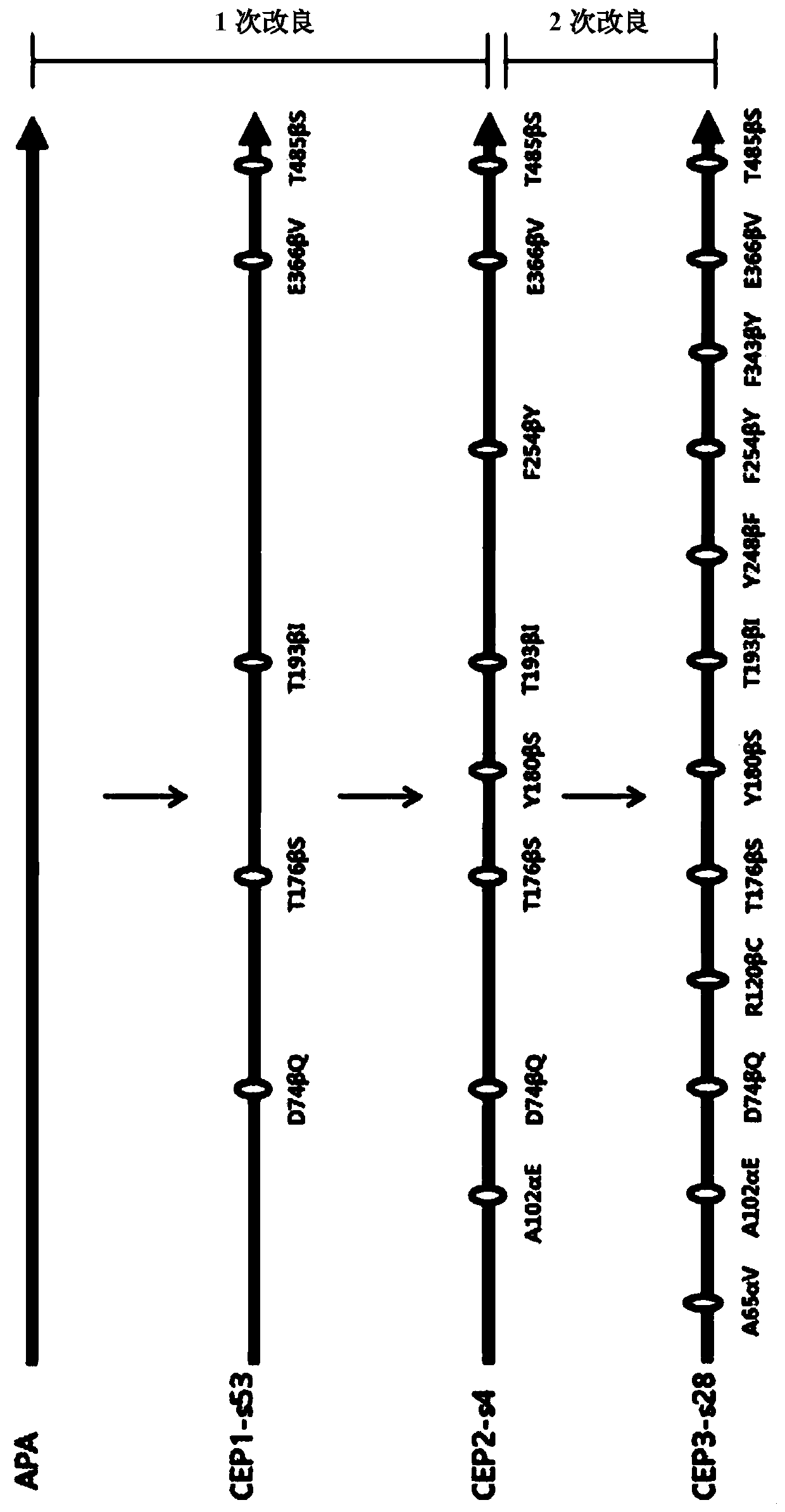
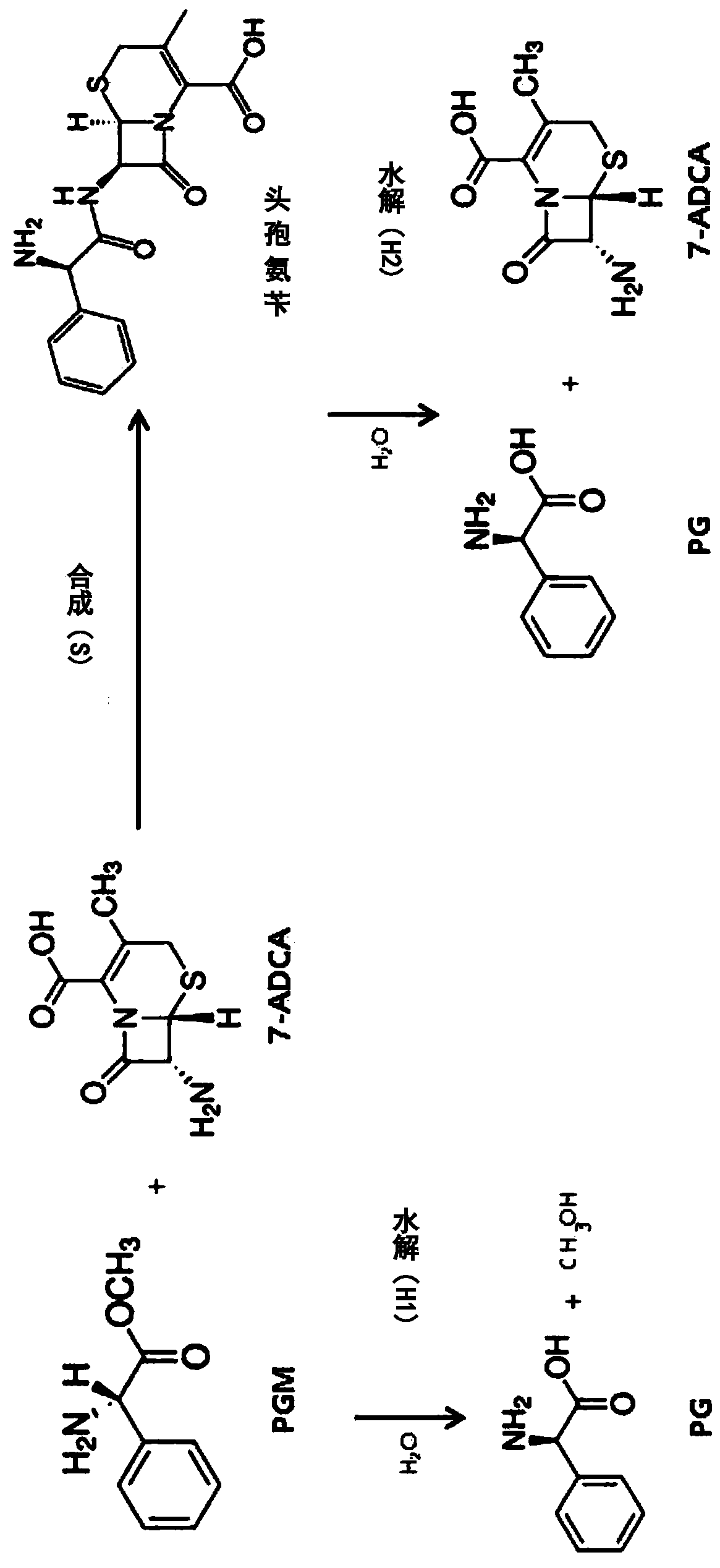
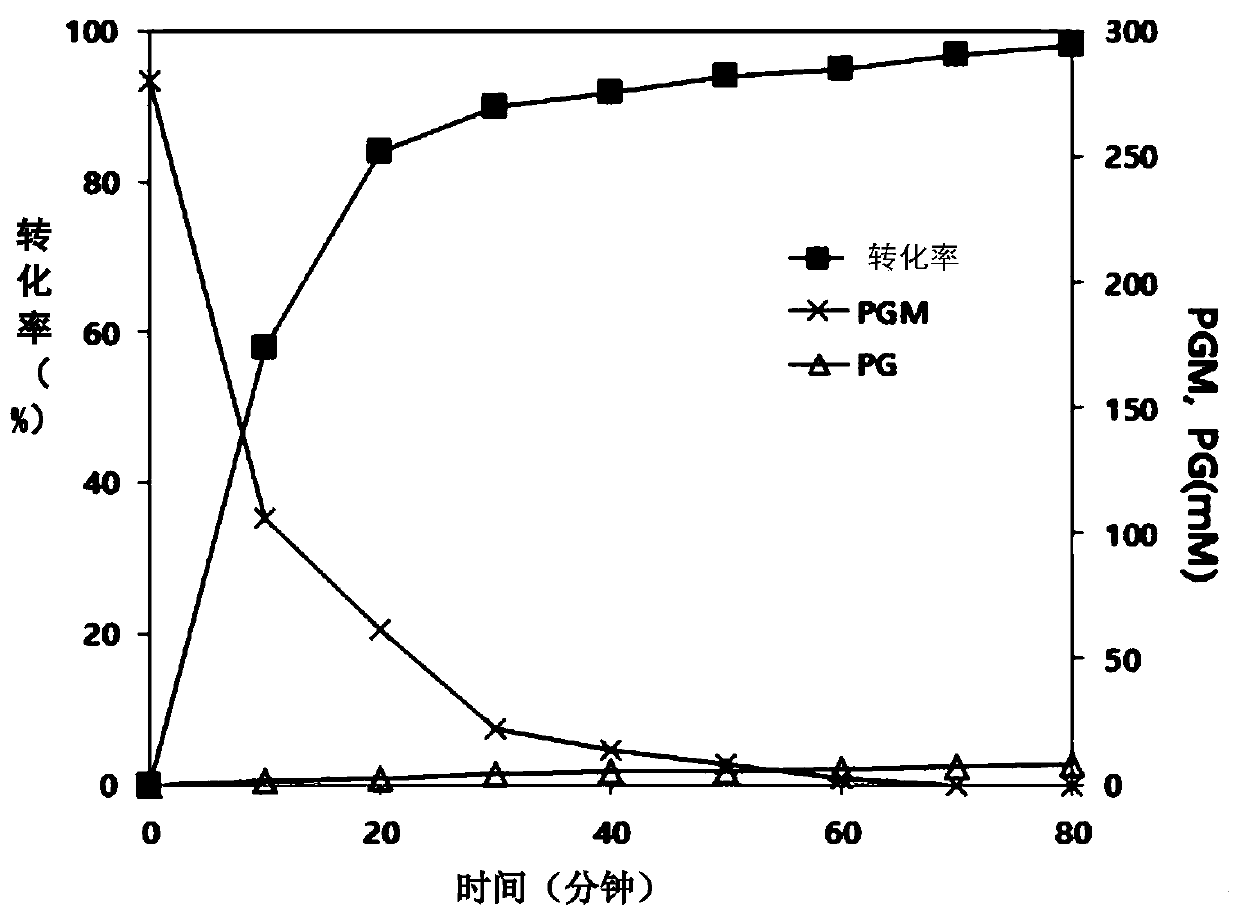
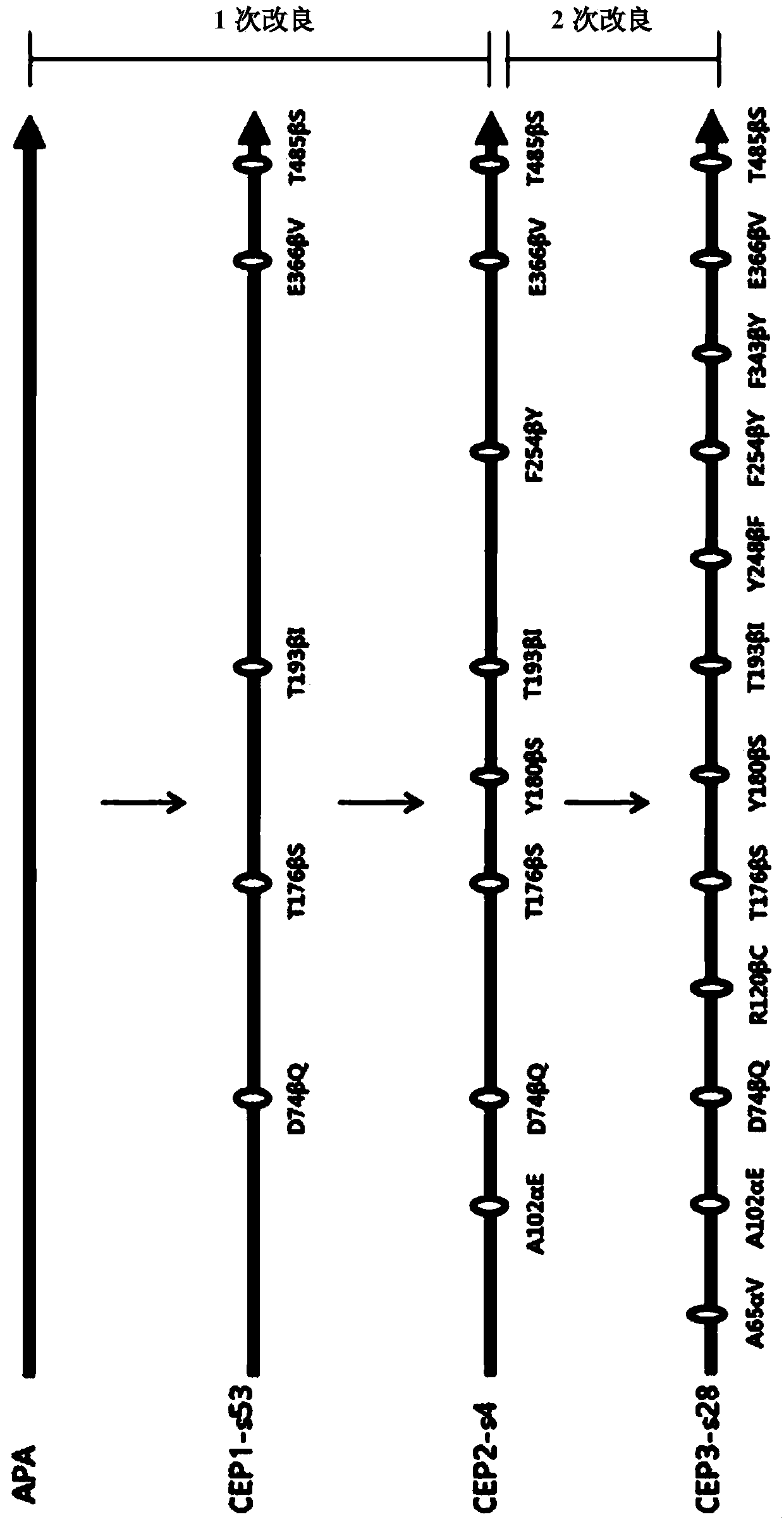
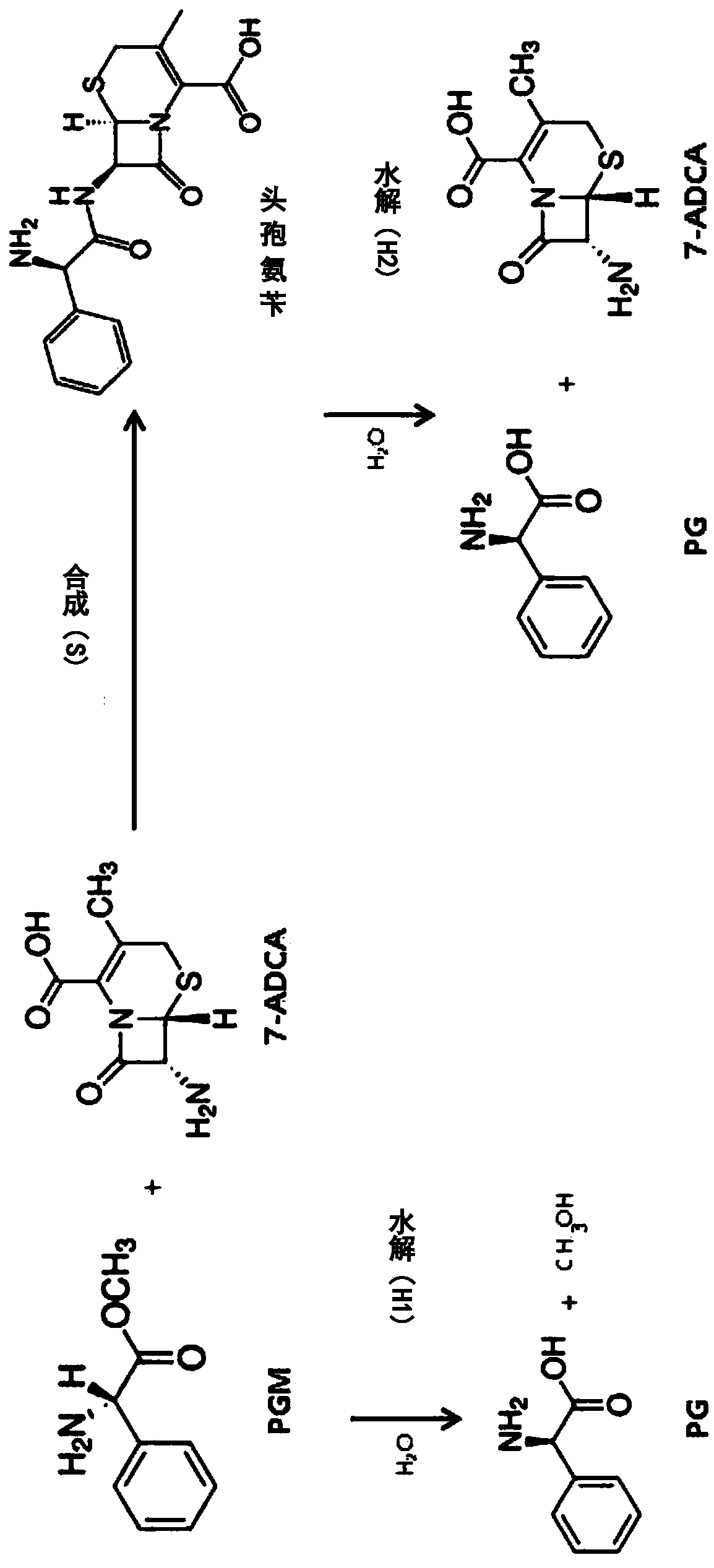
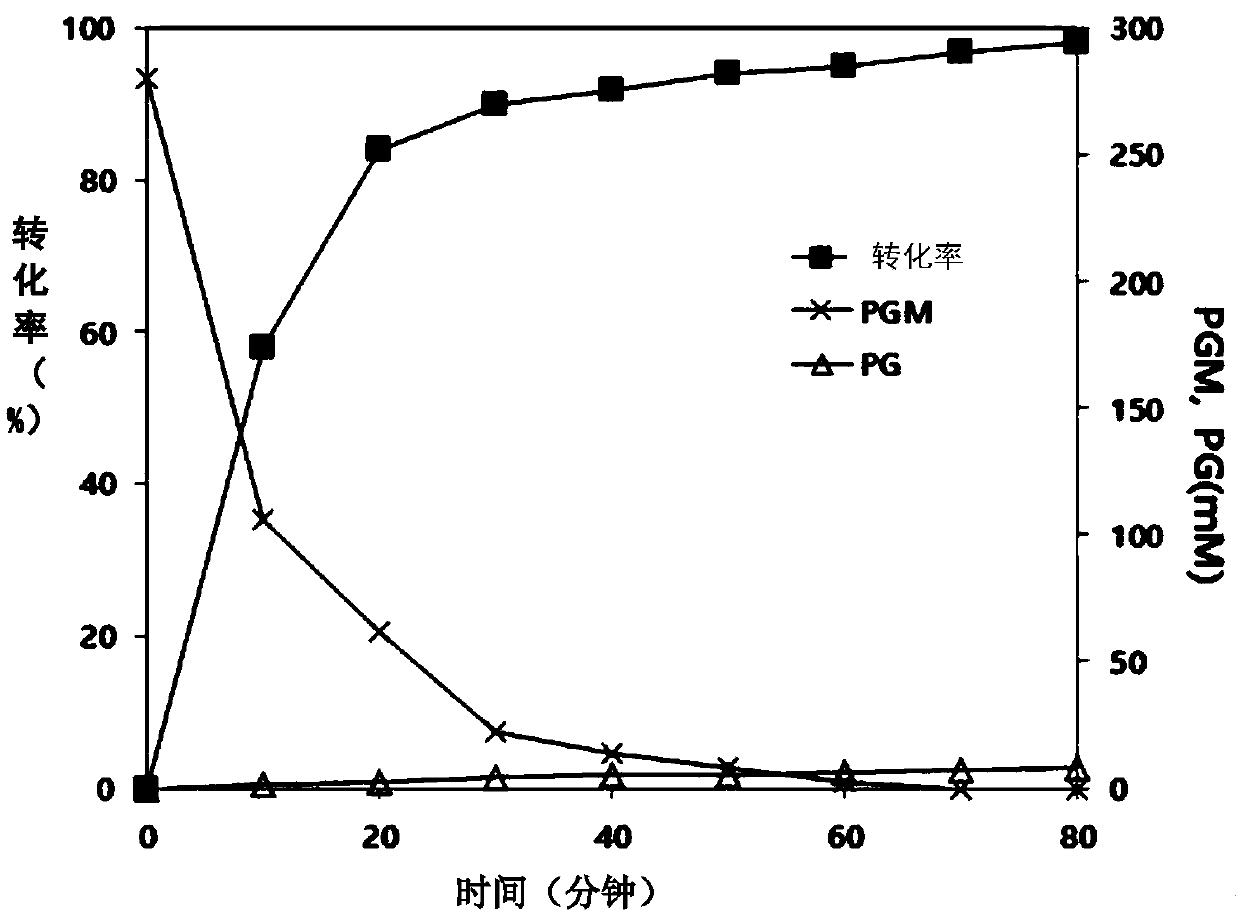
ActiveCN109971743AHigh synthetic activityIncrease productionBacteriaHydrolasesGenus AchromobacterBeta lactam antibiotic
The present invention relates to mutants of penicillin G acylase from Achromobacter sp. CCM 4824 and uses thereof, and more specifically relates to Mutants of penicillin G acylase in a wild type penicillin G acylase protein sequence including an Alpha subunit shown in SEQ ID NO.1 and a Beta subunit shown in SEQ ID NO.2 and a method of preparing (synthesizing) Beta-Lactam antibiotics by using theMutants of penicillin G acylase, wherein the Mutants of penicillin G acylase select at least one mutation from a population consisting of A65AlphaV, A102AlphaE, A106AlphaT, D74BetaQ, R120BetaC, T176BetaS, Y180BetaS, T193BetaI, Y248BetaF, F254BetaY, T292BetaS, F343BetaY, E366BetaV and T485BetaS as the feature. The Mutants of penicillin G acylase from Achromobacter sp. CCM 4824, having a specific mutation form, are characterized by multiple syntheses with high efficiency for multiple semi-synthesized Beta-Lactam antibiotics.
Owner:艾美科健株式会社
Graphene composite ammonia-hydrogen conversion catalyst and preparation method thereof
PendingCN113351204AReduce dosageReduce loadHydrogen productionHydrogen/synthetic gas productionPtru catalystPhysical chemistry
The invention belongs to the technical field of ammonia-hydrogen conversion, and particularly relates to a graphene composite material ammonia-hydrogen conversion catalyst and a preparation method thereof. The graphene composite material ammonia-hydrogen conversion catalyst comprises a graphene-containing composite oxygen hydride, and a metal-based graphene composite oxygen hydride with catalytic activity is obtained by introducing a metal compound solution with ammonia synthesis activity. The graphene composite material ammonia-hydrogen conversion catalyst prepared by the invention has higher decomposition activity than a metal oxide, and aims at reducing the use amount of Ru, Fe, Co and Ni catalysts and other metal loading, so that the cost of the ammonia synthesis catalyst is reduced, the ammonia-hydrogen conversion catalyst with low metal loading capacity is designed and realized, the economic benefit is reduced, industrial production. and the catalytic activity of ammonia-hydrogen conversion is improved.
Owner:唐亚
Titania powder for honeycomb waste gas treating catalyst, and waste gas treating catalyst
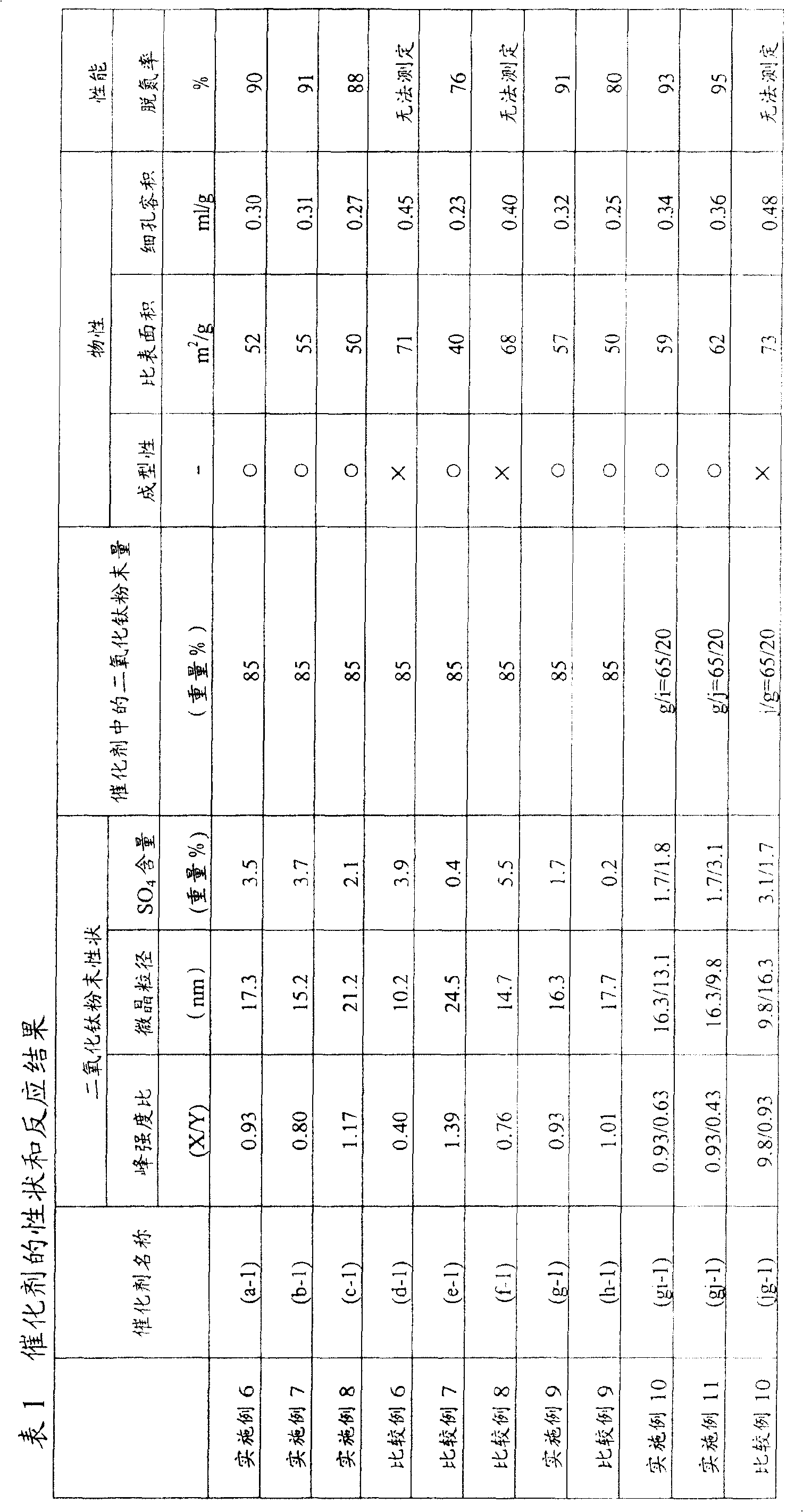
ActiveCN100441287CImprove denitrification activityHigh decomposition activityDispersed particle separationCatalyst activation/preparationHalogenExhaust gas
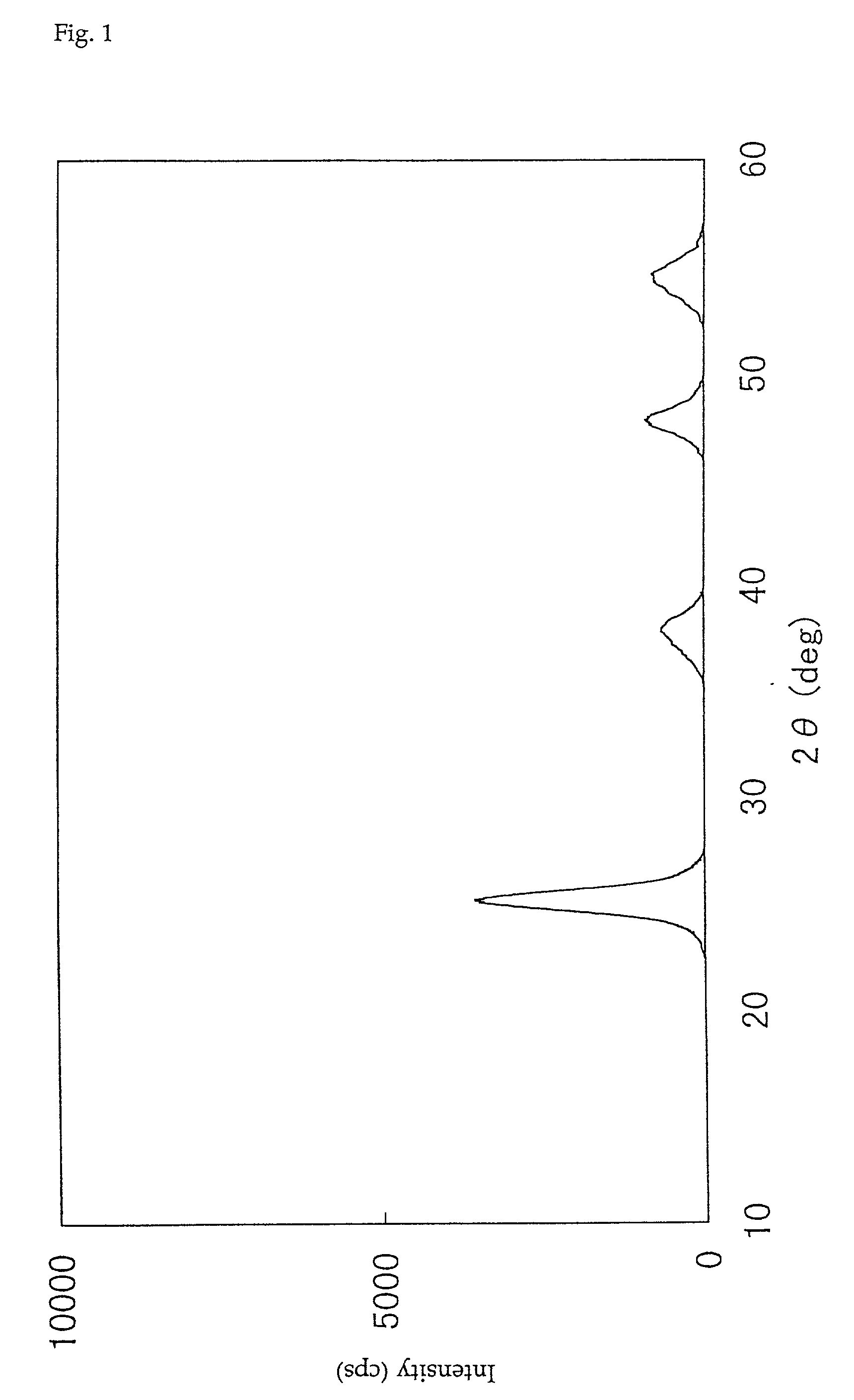
Provided is a raw material for a honeycomb exhaust gas treatment catalyst composed of a powder of titanium dioxide and / or titanium composite oxide, which has excellent extrusion moldability of a honeycomb structure, and a catalyst using the raw material, which has high decomposition activity of an organic halogen compound and Catalyst with high denitrification activity. A titanium dioxide powder for a honeycomb exhaust gas treatment catalyst and a honeycomb exhaust gas treatment catalyst using the titanium dioxide powder. The titanium dioxide powder for honeycomb exhaust gas treatment catalysts is a titanium dioxide powder for honeycomb exhaust gas treatment catalysts composed of titanium dioxide and / or titanium composite oxide, and is characterized by having the following properties: (a) using powder X-ray diffraction The peak intensity ratio of the (101) surface of the anatase titanium dioxide crystal of the titanium dioxide powder relative to the reference sample measured by the method is within a specific range; (b) the crystallite size of the (101) surface of the anatase crystal is within a specific range Within the range of ~22nm; (c) contains 0.3 ~ 5.0% by weight of sulfate (SO4).
Owner:JGC CATALYSTS & CHEM LTD
Mutants of penicillin G acylase from Achromobacter sp. CCM 4824 and uses thereof
InactiveCN109971742AHigh synthetic activityIncrease productionBacteriaHydrolasesGenus AchromobacterBeta lactam antibiotic
The present invention relates to mutants of penicillin G acylase from Achromobacter sp. CCM 4824 and uses thereof, and more specifically relates to Mutants of penicillin G acylase in a wild type penicillin G acylase protein sequence including an Alpha subunit shown in SEQ ID NO.1 and a Beta subunit shown in SEQ ID NO.2 and a method of preparing (synthesizing) Beta-Lactam antibiotics by using the Mutants of penicillin G acylase, wherein the Mutants of penicillin G acylase select at least one mutation from a population consisting of A65AlphaV, A102AlphaE, A106AlphaT, D74BetaQ, R120BetaC, T176BetaS, Y180BetaS, T193BetaI, Y248BetaF, F254BetaY, T292BetaS, F343BetaY, E366BetaV and T485BetaS as the feature. The Mutants of penicillin G acylase from Achromobacter sp. CCM 4824, having a specific mutation form, are characterized by multiple syntheses with high efficiency for multiple semi-synthesized Beta-Lactam antibiotics.
Owner:艾美科健株式会社
Preparation method of nitric acid industrial carbon emission reduction catalyst
PendingCN114160159AHigh activityImprove high temperature stabilityNitrous oxide captureHeterogenous catalyst chemical elementsPtru catalystPhysical chemistry
The invention discloses a preparation method of a nitric acid industrial carbon emission reduction catalyst. The method comprises the following steps: 1, dissolving an active component precursor and an auxiliary agent precursor in deionized water to obtain a transparent dipping solution; 2, adding the cerium dioxide powder into a dipping solution for dipping treatment, and drying to obtain a catalyst precursor; 3, uniformly mixing an adhesive, a pore forming agent and the catalyst precursor, adding water, kneading, and carrying out extrusion molding to obtain a catalyst blank; and 4, drying the catalyst blank, and roasting to obtain the nitric acid industrial carbon emission reduction catalyst. According to the present invention, the types and the proportions of the active components and the auxiliary agent are designed, and the roasting treatment process and the process parameters are controlled so as to obtain the catalyst with the relatively dispersed active components and the high crystallinity, such that the number of the effective active centers in the catalyst is increased so as to improve the activity of the catalyst, and the catalyst has better high-temperature stability and is suitable for N2O emission reduction in the nitric acid production process.
Owner:XIAN ORIGIN CHEM TECH
Composite nano-photo-catalyst used for purifying air
InactiveCN1269568CExtended service lifeHigh activityDispersed particle separationCatalyst activation/preparationBenzenePhoto catalytic
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
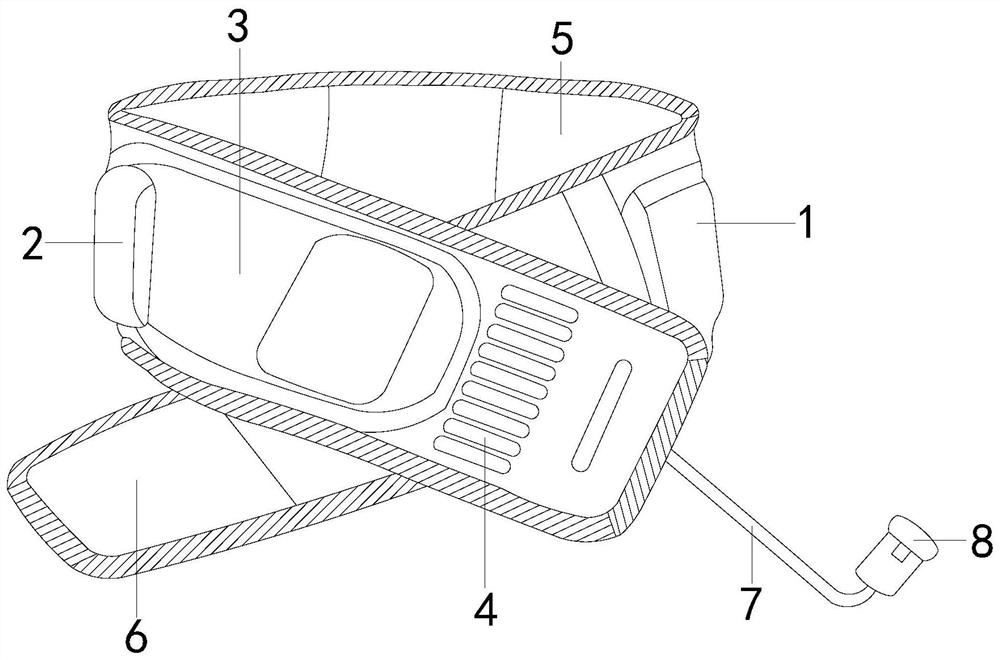
A portable microbaric oxygen chamber host
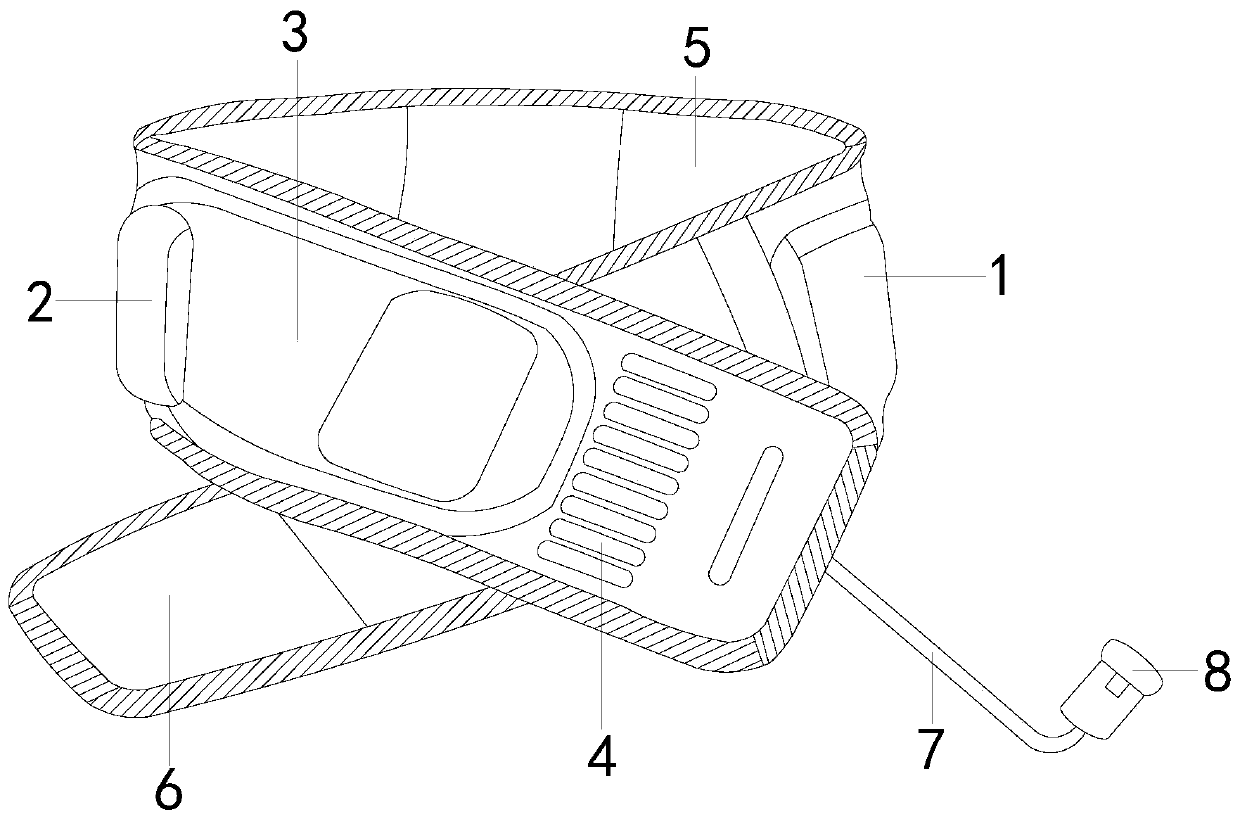
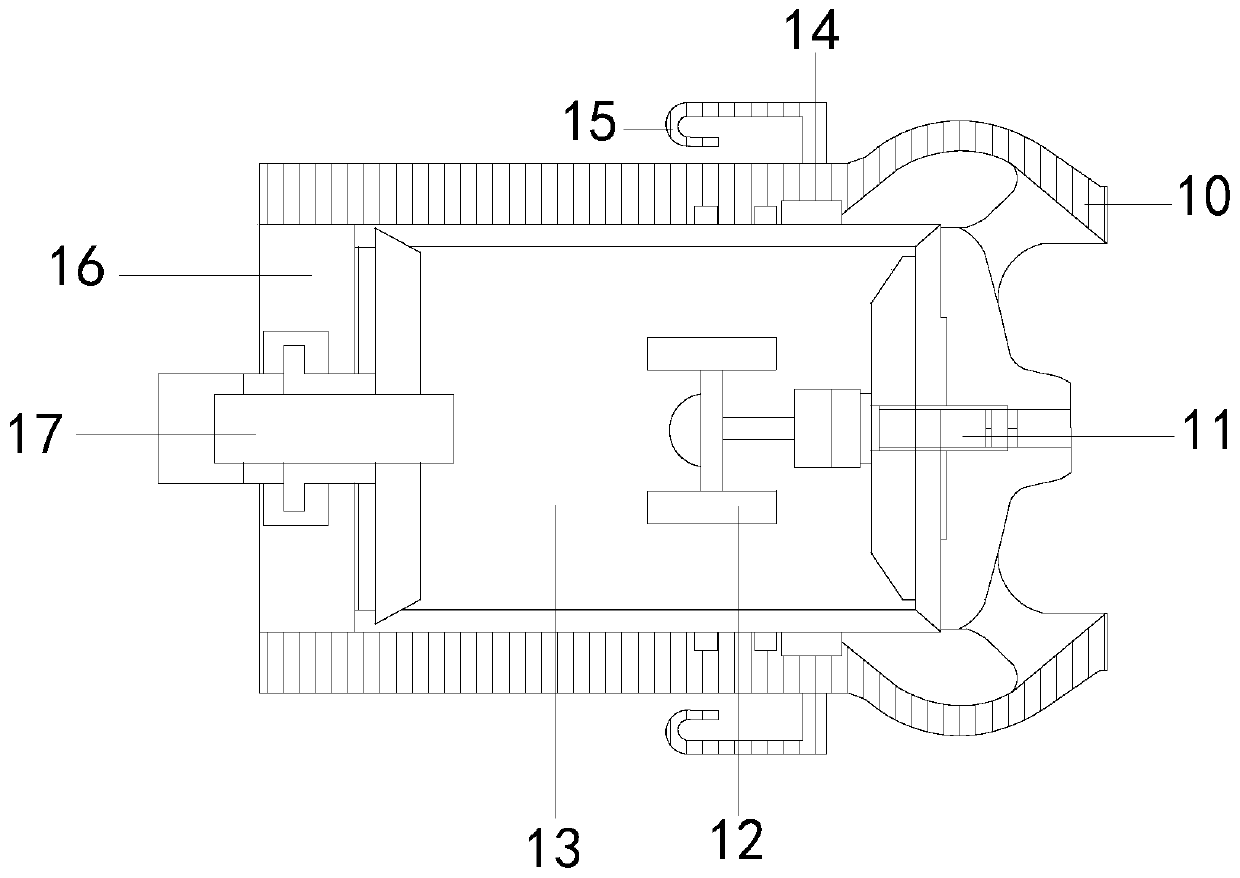
ActiveCN110464574BSolve the problem of releasing high-purity oxygenSpeeds up scab healingBreathing protectionElectrotherapyEngineeringCatheter
The invention discloses a portable micro-pressure oxygen chamber main unit, the structure of which includes: a strap main body, a main unit bag, a main unit, a ventilating groove, a wool surface layer, a hook surface layer, a catheter, and an oxygen output tank. The beneficial effects of the invention are as follows: The structure is equipped with an oxygen output tank to solve the problem of how to maintain the release of oxygen to the wound during the long-term treatment process. Using the hook block can enhance the anti-falling performance of the device, and its hook structure can prevent the elastic cord from breaking off the hook block. Open, the high-purity oxygen produced by the main engine of the enhanced oxygen chamber can be continuously and stably released to the wound surface within a certain period of time, thereby accelerating wound healing and scab formation.
Owner:广州埃珥赛璞医疗设备有限公司
A method for efficiently decomposing and recovering valuable metals in copper slag
ActiveCN107955878BRealize magnetic separation recoveryLower decomposition temperatureMagnetic separationAir atmosphereIronstone
The invention discloses a method for efficiently decomposing and recycling valuable metal in copper slag. According to the method, after the copper slag and cryolite are mixed and ball-milled, the mixture is arranged in an air atmosphere for calcination; calcination products are crushed and undergo magnetic separation, and then magnetite is obtained. By the adoption of the method for efficiently decomposing and recycling the valuable metal in the copper slag, iron resources incapable of being reused in the copper slag can be directionally regulated and controlled to be efficiently converted into the magnetite under mild conditions, reduction and reclamation of the copper slag are achieved, the copper slag tail end single-pass treatment problem, the national high-grade iron ore resource starvation problem, and the environmental pollution problem are solved, and a green sustainable development road is opened up for treatment of the copper slag.
Owner:CENT SOUTH UNIV
Portable micro-pressure oxygen chamber main machine
ActiveCN110464574ASolve the problem of releasing high-purity oxygenSpeeds up scab healingBreathing protectionElectrotherapyWound healingSurface layer
The invention discloses a portable micro-pressure oxygen cabin main machine, the structure of which comprises a suspender main body, a main machine bag, a main machine, a ventilation groove, a wool surface layer, a hook surface layer, a conduit and an oxygen output tank. The beneficial effects of the invention are as follows: the oxygen output tank is arranged on the structure to solve the problemof how oxygen can maintain oxygen delivery release to wounds in a long-term treatment process, the hook block can enhance the anti-falling performance of equipment, and the hook structure of the portable micro-pressure oxygen cabin main machine can prevent elastic ropes from being disconnected from the hook block, thereby enhancing the continuous and stable release of high-purity oxygen producedby the oxygen cabin main machine to wound surfaces within a certain period of time, thereby speeding up wound healing scabbing.
Owner:广州埃珥赛璞医疗设备有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com