Catalyst for catalytic decomposition of nitrous oxide and preparation method of catalyst
A technology for nitrous oxide and catalytic decomposition, which is applied in the fields of nitrous oxide capture, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. Activity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
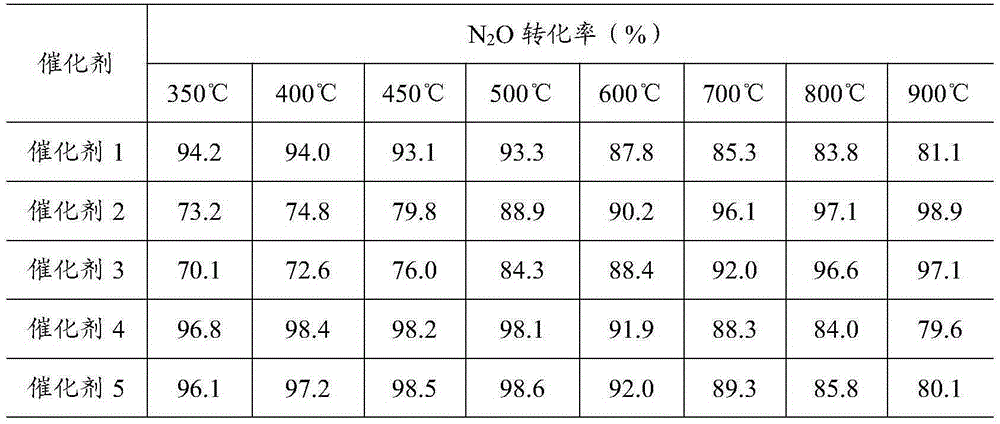
Examples
Embodiment 1
[0074] Embodiment 1: prepare catalyst of the present invention
[0075] The implementation steps of this embodiment are as follows:
[0076] A. Preparation of impregnation solution
[0077] According to the mass ratio of the active component precursor to the auxiliary agent precursor of 0.4:1.0, the cobalt acetate active component precursor and the platinum nitrate auxiliary agent precursor are dissolved in water to obtain a transparent impregnating solution; then
[0078] B. Preparation of catalyst precursor
[0079] At room temperature, according to the ratio of the ceria carrier in grams to the impregnating solution in milliliters is 100:100, allowing the ceria carrier to be impregnated in the impregnating solution obtained in step A for 2.0h to obtain a paste solid, Then dry the paste solid at a temperature of 80° C. for 24 hours, and then grind the obtained dry powder to a particle size of less than 10 μm to obtain a catalyst precursor;
[0080] C. Catalyst molding
...
Embodiment 2
[0086] Embodiment 2: prepare catalyst of the present invention
[0087] The implementation steps of this embodiment are as follows:
[0088] A. Preparation of impregnation solution
[0089] According to the mass ratio of the active component precursor and the auxiliary agent precursor 8.0:0.01, the active component precursor and the palladium nitrate auxiliary agent precursor are dissolved in cobalt acetylacetonate and aluminum acetate (the weight ratio of cobalt and aluminum is 3:1) aqueous solution to obtain a transparent impregnating solution; then
[0090] B. Preparation of catalyst precursor
[0091] At room temperature, according to the ratio of the ceria carrier in grams to the impregnating solution in milliliters is 100:100, allowing the ceria carrier to be impregnated in the impregnating solution obtained in step A for 1.0 h to obtain a paste solid, Then dry the paste solid at a temperature of 120° C. for 12 hours, and then grind the obtained dry powder to a partic...
Embodiment 3
[0098] Embodiment 3: prepare catalyst of the present invention
[0099] The implementation steps of this embodiment are as follows:
[0100] A. Preparation of impregnation solution
[0101] Dissolve cobalt nitrate, aluminum nitrate (cobalt to aluminum weight ratio 4:1) active component precursor and rhodium nitrate auxiliary precursor in aqueous solution according to the mass ratio of active component precursor to auxiliary agent precursor 2.5:0.1 , a transparent impregnating solution was obtained; then
[0102] B. Preparation of catalyst precursor
[0103] At room temperature, according to the ratio of the ceria carrier in grams to the impregnation solution in milliliters is 100:30, the ceria carrier is immersed in the impregnation solution obtained in step A for 1.0h to obtain a paste solid, Then dry the paste solid at a temperature of 90° C. for 20 hours, and then grind the obtained dry powder to a particle size of less than 10 μm to obtain a catalyst precursor;
[0104] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com