Preparation method of supported medium and high temperature denitration catalyst
A denitrification catalyst, medium and high temperature technology, applied in the direction of catalyst activation/preparation, molecular sieve catalyst, chemical instruments and methods, etc., can solve the problem of poor catalytic activity of the catalyst, achieve good environmental protection and social benefits, improve dispersion, and not easy to powder effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
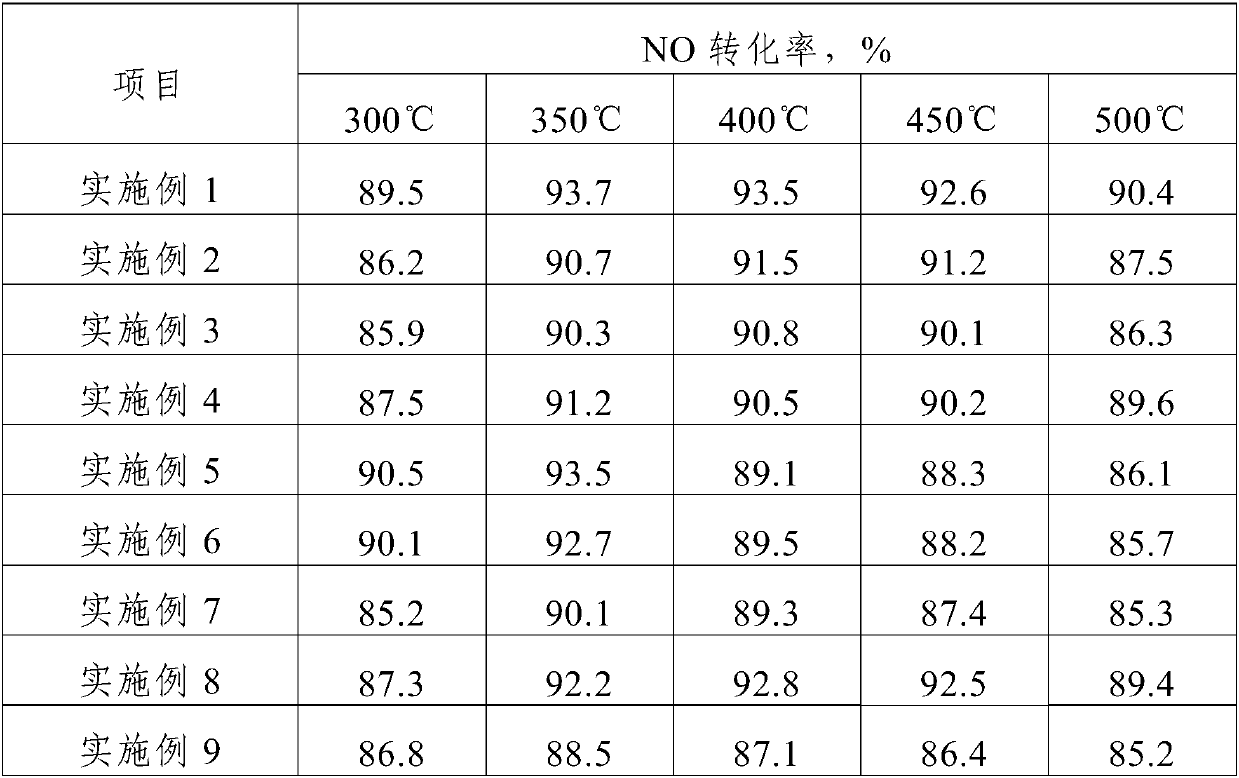
Embodiment 1
[0022] The method in this embodiment includes the following steps:
[0023] Step 1, the soluble active metal component precursor salt and the soluble auxiliary metal component precursor salt are all dissolved in deionized water to obtain a transparent impregnation solution; the soluble active metal component precursor salt is ferrous chloride; the soluble The auxiliary metal component precursor salt is tin tetrachloride; the quality of the active metal elemental Fe in the soluble active metal component precursor salt, the auxiliary metal element Sn in the soluble auxiliary metal component precursor salt and deionized water The ratio is 2.5:1:50;
[0024] Step 2, placing the molecular sieve carrier in the impregnation solution obtained in step 1 and immersing it for 12 hours, and drying it for 16 hours at a temperature of 110°C after filtering to obtain a catalyst precursor; the ratio of the mass of the molecular sieve carrier to the volume of the impregnation solution is 100:...
Embodiment 2
[0027] The method of the present embodiment comprises the following steps:
[0028] Step 1. Both the soluble active metal component precursor salt and the soluble additive metal component precursor salt are dissolved in deionized water to obtain a transparent impregnation solution; the active metal elemental Fe soluble additive in the soluble active metal component precursor salt The mass ratio of the auxiliary metal elemental Cr in the metal component precursor salt to deionized water is 5:2.5:75; the soluble active metal component precursor salt is ferric chloride; the soluble metal component precursor salt is chromium nitrate;
[0029] Step 2, placing the molecular sieve carrier in the impregnating solution obtained in step 1 for 24 hours, filtering and drying at 80°C for 24 hours to obtain a catalyst precursor; the ratio of the mass of the molecular sieve carrier to the volume of the impregnating solution is 100:75, wherein the unit of the mass of the molecular sieve carr...
Embodiment 3
[0032] The method of the present embodiment comprises the following steps:
[0033] Step 1. Both the soluble active metal component precursor salt and the soluble additive metal component precursor salt are dissolved in deionized water to obtain a transparent impregnation solution; the active metal elemental Fe soluble additive in the soluble active metal component precursor salt The mass ratio of the additive metal elemental Zr in the metal component precursor salt to deionized water is 10:0.5:100; the precursor salt of the soluble active metal component is iron nitrate; the precursor salt of the metal component of the soluble additive is Zirconium nitrate;
[0034] Step 2. Place the molecular sieve carrier in the impregnation solution obtained in step 1 and immerse it for 18 hours, filter and dry it for 12 hours at a temperature of 120° C. to obtain a catalyst precursor; the ratio of the mass of the molecular sieve carrier to the volume of the impregnation solution is 100:1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| denitrification rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com