Ammonia decomposition catalyst
A catalyst and ammonia decomposition technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc. issues such as sexual decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
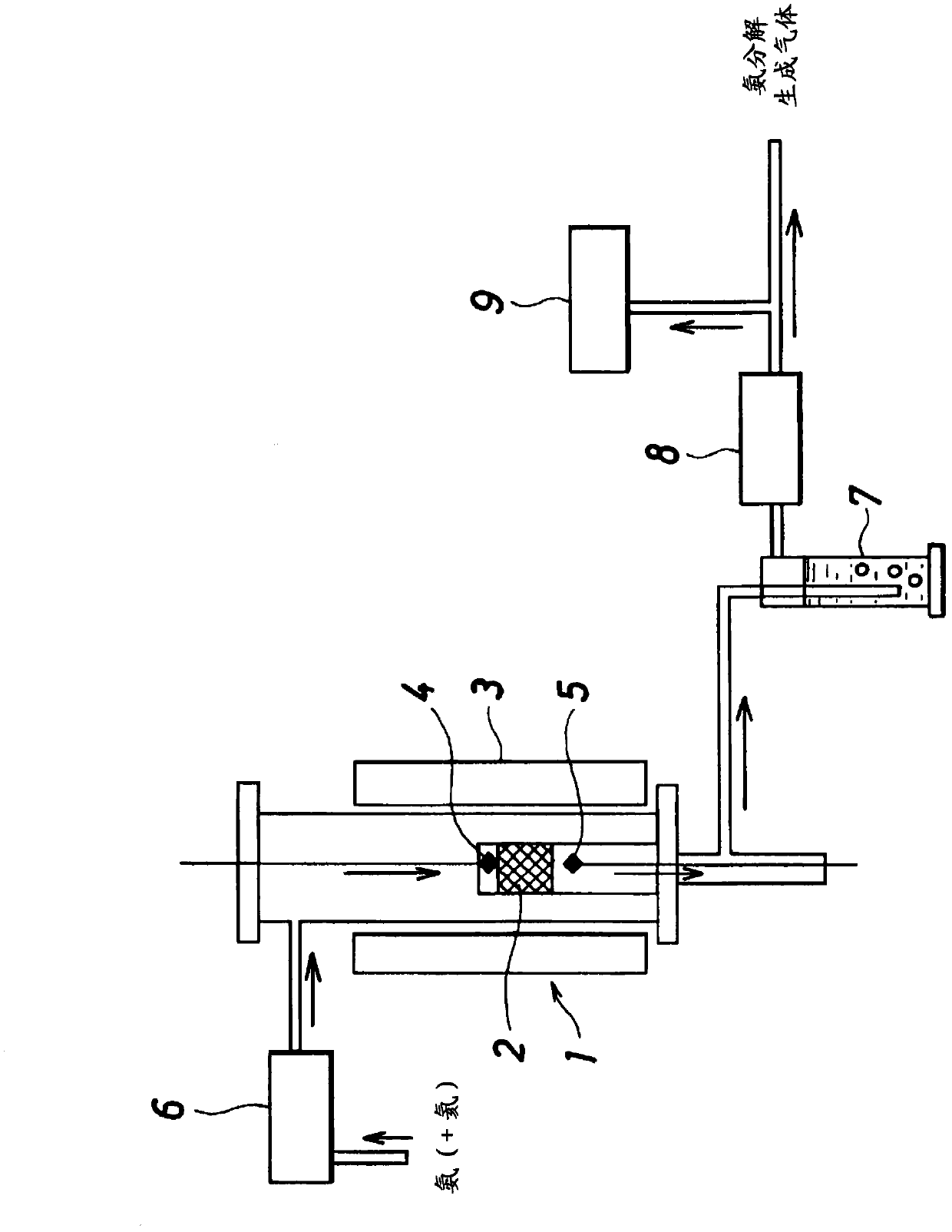
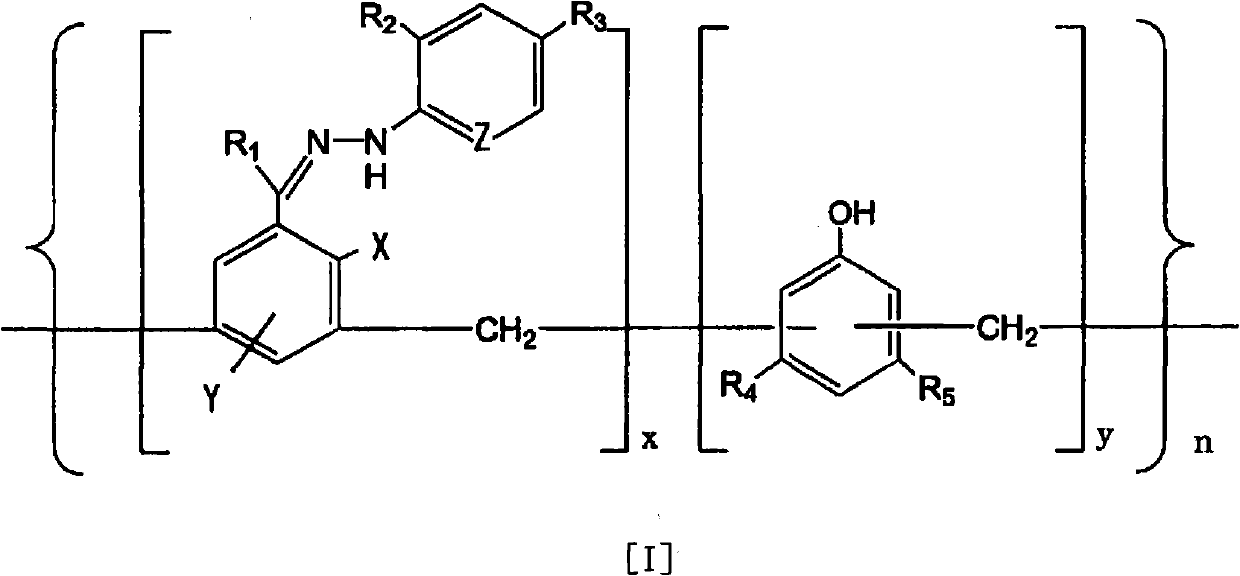
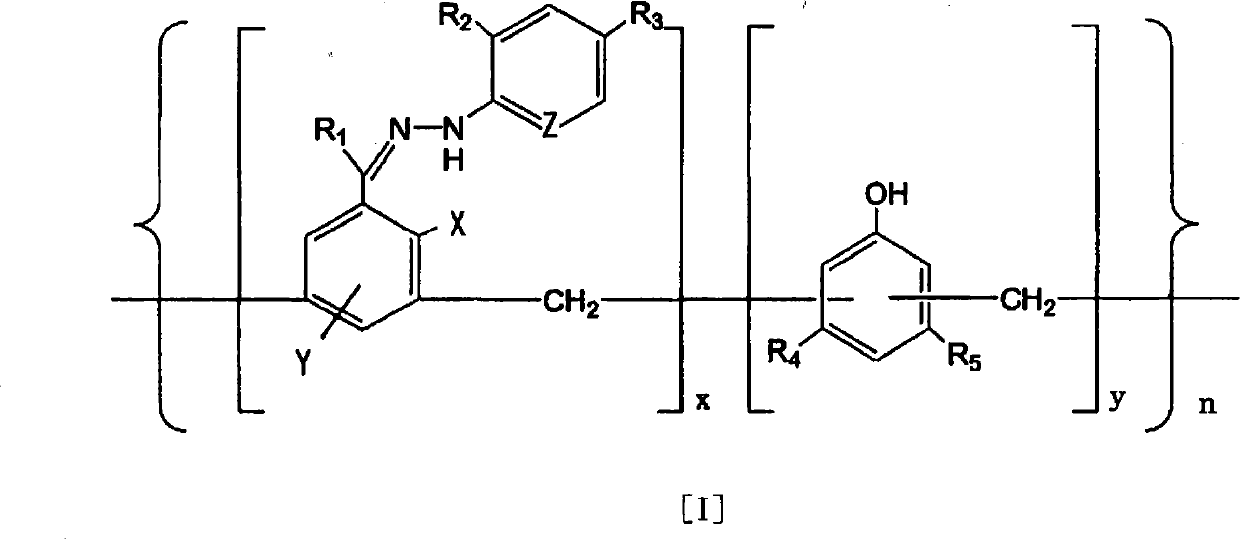
[0093] i) Suspend 4-[1-[(2,4-dinitrophenyl)hydrazono]ethyl}-1,3-benzenediol (compound 1) in the form of monomer in ion-exchange water , to the resulting suspension were added phenol and 40 wt% aqueous formaldehyde at room temperature. These addition amounts were hydrazone compound:phenol:40wt% formaldehyde aqueous solution=1g:0.5g:0.5ml. NaOH was further added to the above mixture, the whole solution was stirred, and reflux was performed at 110° C. for 8 hours. The amount of NaOH added was hydrazone compound:NaOH=32g:1g. The solid matter obtained as described above was collected by filtration, washed several times with ion-exchanged water, and then adjusted to pH 7 in ion-exchanged water. Thereafter, after filtering and washing, drying was carried out at 60°C for 2 to 3 hours to obtain 27.5 g of a polymer having a molecular weight of 1,000 to 500,000.
[0094] ii) The above polymer was suspended in 100 ml of a 10 g / l aqueous ruthenium chloride solution, stirred for 2 hours,...
Embodiment 2~10
[0097] The following compounds are used in monomeric form:
[0098] 2-{1-[(2,4-dinitrophenyl)hydrazono]ethyl}-1-phenol (compound 2),
[0099] 4-{1-[(2,4-dinitrophenyl)hydrazono]ethyl}-1-phenol (Compound 3),
[0100] 3-{1-[(2,4-dinitrophenyl)hydrazono]ethyl}-1,4-benzenediol (compound 4),
[0101] 4-{1-[(2,4-dinitrophenyl)hydrazono]methyl}-1,3-benzenediol (Compound 5),
[0102] 4-{1-[(4-nitrophenyl)hydrazono]ethyl}-1,3-benzenediol (compound 6),
[0103] 4-{1-[(2-nitrophenyl)hydrazono]ethyl}-1,3-benzenediol (compound 7),
[0104] 4-{1-[(2,4-dichlorophenyl)hydrazono]ethyl}-1,3-benzenediol (compound 8),
[0105] 4-{1-[(phenyl)hydrazono]ethyl}-1,3-benzenediol (compound 9),
[0106] 4-{1-[(2-pyridyl)hydrazono]ethyl}-1,3-benzenediol (compound 10),
[0107] Except that, the same operation as in Example 1 was carried out to obtain a catalyst.
Embodiment 11
[0109] i) suspending 4-{1-[(2,4-dinitrophenyl)hydrazono]ethyl}1,3-benzenediol (compound 1) in ion-exchanged water in the form of a monomer, To the resulting suspension were added phenol and 40 wt% aqueous formaldehyde at room temperature. These addition amounts were hydrazone compound:phenol:40wt% formaldehyde aqueous solution=1g:0.5g:0.5ml. NaOH was further added to the above mixture, the whole solution was stirred, and reflux was performed at 110° C. for 8 hours. The amount of NaOH added was hydrazone compound:NaOH=32g:1g. The solid matter obtained as described above was collected by filtration, washed several times with ion-exchanged water, and its pH was adjusted to 7 in ion-exchanged water. Thereafter, after filtering and washing, drying was carried out at 60°C for 2 to 3 hours to obtain 27.5 g of a polymer having a molecular weight of 1,000 to 500,000.
[0110] ii) The above polymer was suspended in 100 ml of a 10 g / l aqueous ruthenium chloride solution, stirred for 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com