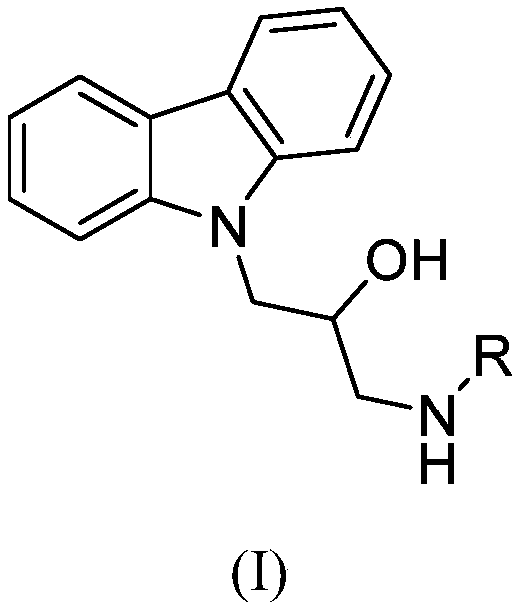
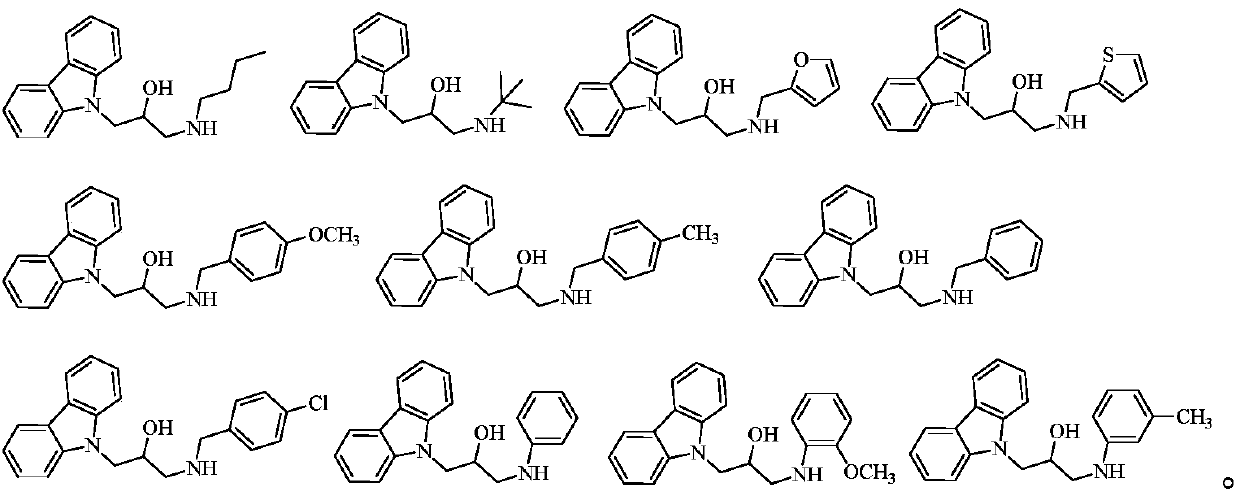
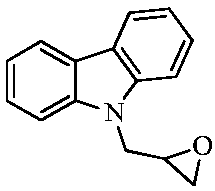
Preparation method for carbazolyl isopropanolamine derivative and application thereof
A technology of carbazolyl isopropanolamine and alkyl, which is applied in the field of preparation of carbazolyl isopropanolamine derivatives, and can solve the problems of environmental pollution, poor prevention effect and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of 1-(benzylamino)-3-(9H-carbazolyl)-2-hydroxypropanol
[0053] Add 9-(2-methyloxyethylene)-9H-carbazole (0.90mmol) and potassium carbonate (0.90mmol) into 5mL isopropanol solution dissolved with benzylamine (1.80mmol), and react at 60°C for 6 hours After stopping the reaction, desolvation, column chromatography (eluent CH 2 Cl 2 :CH 3 OH=200:1) to obtain a white solid with a yield of 53.7%.
[0054] The structures, H-NMR and C-NMR data of some synthesized compounds containing carbazolyl isopropanolamine structures are shown in Table 1, and their physical and chemical properties are shown in Table 2.
[0055] H NMR spectrum and carbon spectrum data of some compounds in Table 1
[0056]
[0057]
[0058]
[0059] Table 2 Physicochemical properties of some target compounds
[0060]
[0061]
[0062] Pharmacological Example 1:
[0063] The inhibitory rate of the target compounds to plant pathogenic bacteria was tested by turbidim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com