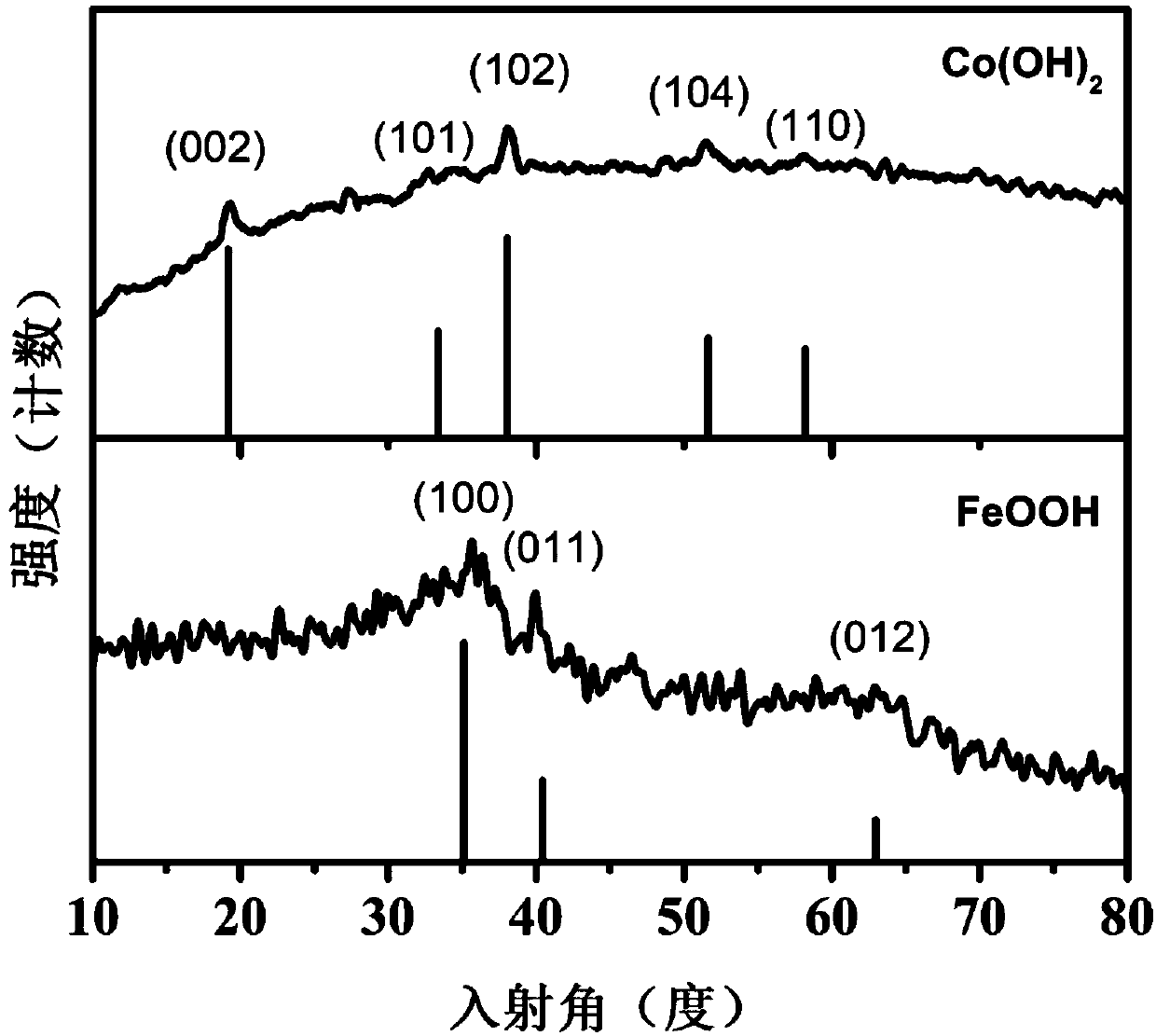
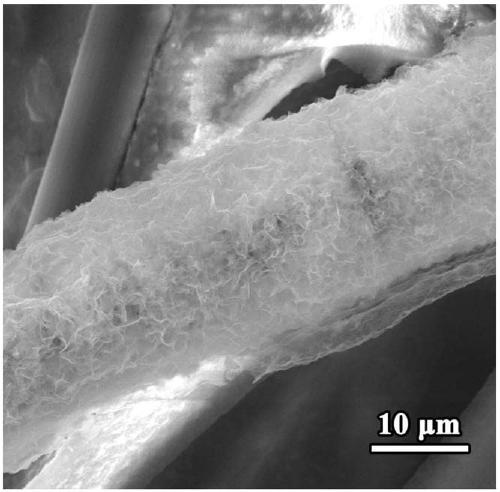
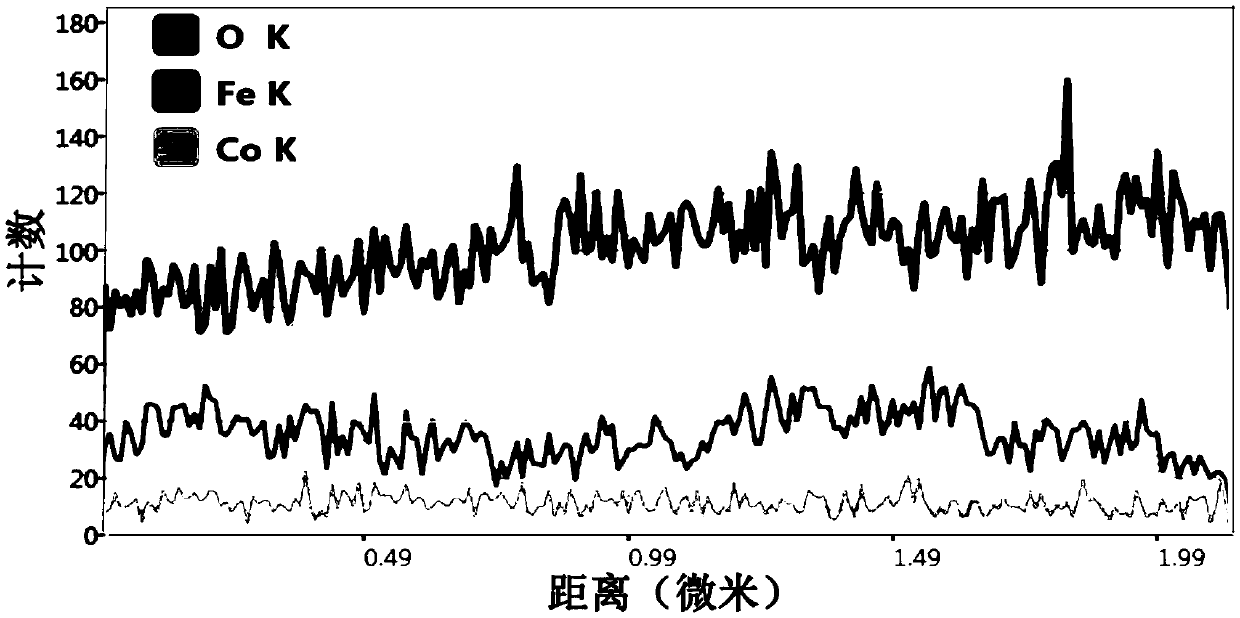
Oxygen evolution reaction FeOOH/Co(OH)2 composite electrocatalyst preparation method
An oxygen evolution reaction and electrocatalyst technology, applied in electrodes, circuits, electrical components, etc., can solve the problems of poor dispersion and poor electrocatalytic performance of oxygen evolution reaction, and achieve convenient operation, improved electrocatalytic performance, and high electrocatalytic performance. and electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1: Dissolve an appropriate amount of cobalt nitrate in a certain volume of deionized water, stir at 25°C for 15 minutes, and use the prepared cobalt nitrate solution (0.1mol / L) as the electrolyte;
[0033]Step 2: Cut carbon fiber paper with a thickness of 0.3mm into 1*2cm long strips and heat in a water bath at 60°C for 2 hours in a 1mol / L potassium hydroxide solution, then ultrasonically in absolute ethanol and deionized water for 30 minutes, take out Finally, dry in an oven at 60°C for 3 hours;
[0034] Step 3: Use the electrode holder to clamp the carbon fiber paper treated in step 2 as the working electrode, the platinum-coated titanium mesh (2*3cm) as the counter electrode, the saturated calomel electrode as the reference electrode, and the cobalt nitrate solution in step 1 as the electrolyte , carry out step-by-step constant voltage deposition; the first deposition voltage is -1.1V, the deposition time is 100s, and then the second step deposition is performed...
Embodiment 2
[0041] Other steps are the same as in Example 1, except that the number of deposition circles in step 3 is changed from 1 circle to 2 circles.
Embodiment 3
[0043] Other steps are the same as in Example 1, except that the number of deposition circles in step 3 is changed from 1 circle to 3 circles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com