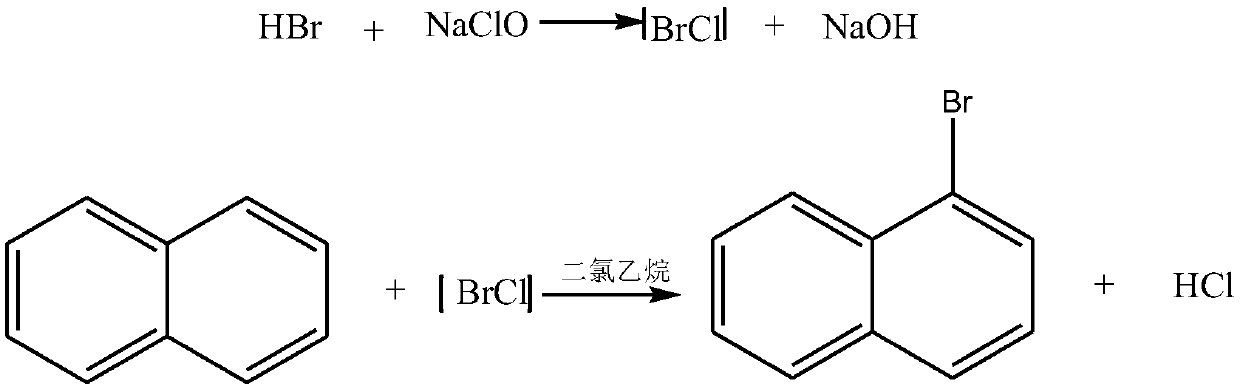
Preparation method of 1-bromonaphthalene
A technology of brominated naphthalene and hydrobromic acid, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of high production energy consumption, violent reaction, and large energy consumption, and achieve less reaction by-products , The effect of simple reaction device and low production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The method that present embodiment prepares 1-bromonaphthalene is as follows:
[0023]
[0024] (1) Get the refined naphthalene of 1mol, be fully dissolved in the ethylene dichloride, then in the stirring process, the hydrobromic acid of 1.1mol is mixed in the solution of ethylene dichloride and naphthalene to form a mixed solution;
[0025] (2) In the process of constant stirring, gradually drop 1.05mol sodium hypochlorite and the above mixed solution under the condition of 30°C;
[0026] (3) After the dropwise addition is completed, stir and keep warm for 60 minutes, then let stand to separate layers, separate the oil phase layer, and then wash it twice to obtain the crude product of the oil phase;
[0027] (4) drying, and then fractional distillation under reduced pressure to obtain 1-bromonaphthalene (yield 95%).
Embodiment 2
[0029] The method that present embodiment prepares 1-bromonaphthalene is as follows:
[0030] (1) Get the refined naphthalene of 1mol, fully dissolve in the ethylene dichloride, then in the stirring process, the hydrobromic acid of 1.05mol is mixed in the solution of ethylene dichloride and naphthalene to form a mixed solution;
[0031] (2) In the process of constant stirring, gradually drop 1.1mol sodium hypochlorite and the above mixed solution under the condition of 25°C;
[0032] (3) After the dropwise addition is completed, stir and insulate for 90 minutes, then stand for stratification, separate the oil phase layer, and then wash it twice to obtain the crude product of the oil phase;
[0033] (4) drying, and then fractional distillation under reduced pressure to obtain 1-bromonaphthalene (yield 96%).
Embodiment 3
[0035] The method that present embodiment prepares 1-bromonaphthalene is as follows:
[0036] (1) Get the refined naphthalene of 1mol, be fully dissolved in the ethylene dichloride, then in the stirring process, the hydrobromic acid of 1.1mol is mixed in the solution of ethylene dichloride and naphthalene to form a mixed solution;
[0037] (2) In the process of constant stirring, gradually drop 1.1mol sodium hypochlorite and the above mixed solution under the condition of 28°C;
[0038] (3) After the dropwise addition is completed, stir and insulate for 70 minutes, then let stand for stratification, separate the oil phase layer, and then wash it twice to obtain the crude product of the oil phase;
[0039] (4) drying, and then fractional distillation under reduced pressure to obtain 1-bromonaphthalene (yield 95%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com