Method for preparing clinical-level adipose-derived stem cells
An adipose stem cell and adipose tissue technology, applied in the field of cell culture technology and biological therapy, can solve the problems of increased risk, low vitality, and non-compliance with clinical application requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a method for preparing clinical-grade adipose stem cells, the steps are as follows:
[0025] (1) The adipose tissue is washed with double anti-salt water, shredded, and impurities, grease and other substances are removed;
[0026] (2) Repeated injection of chylodious adipose tissue through a syringe to shorten the digestion time and increase the number and vitality of stem cells;
[0027] (3) Digest the adipose tissue with a mixed enzyme of type I collagenase, hyaluronidase, deoxyribonuclease I, and trypsin, filter, wash, and discard the supernatant to obtain adipose stem cells.
[0028] Further, the method of the present invention also includes the following steps:
[0029] (4) Inoculate the adipose-derived stem cells in a-MEM medium for culture, which contains cell nutrient supplements, EGF, bFGF, TGF, HGF, PDGF, and does not contain exogenous serum and other non-conforming clinical-grade culture reagents.
[0030] As a preferred solution, the...
Embodiment 1
[0036] Example 1: Isolation and Preparation of Adipose Stem Cells
[0037] Adipose stem cells were isolated and prepared by the following steps:
[0038] 1) Obtain adipose tissue from cosmetic liposuction fluid or surgical fat mass, and wash the adipose tissue with double-resistant saline solution containing penicillin and streptomycin mixture, wherein the working concentration of penicillin is 100U / ml, and the working concentration of streptomycin is 0.1mg / ml, shredded to remove impurities such as grease;
[0039] 2) Take out 30ml from the adipose tissue obtained in step 1) and add it to a 50ml syringe, and repeatedly inject the syringe for 3 minutes, 30 times per minute, to make the adipose tissue chylolysis; wherein, the duration of the repeated injection of the syringe is 1 minute to 5 minutes, 10-30 times per minute; if the time is too short, the adipose tissue will not be fully emulsified; if the time is too long, the stem cells will be damaged and the recovery effect w...
Embodiment 2
[0043] Embodiment 2: Adipose stem cell bacteria, fungus, endotoxin, mycoplasma detection prepared by the present invention
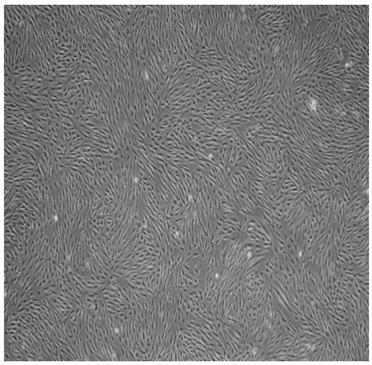
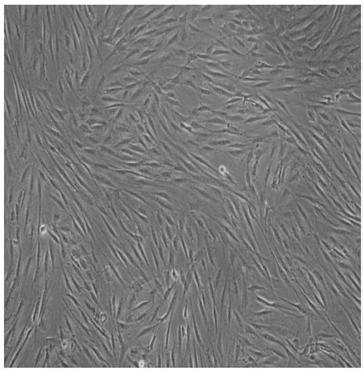
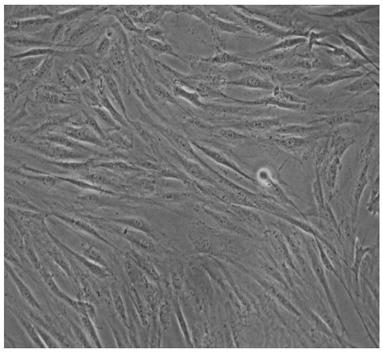
[0044] The cell growth state of the adipose stem cells prepared by the method of Example 1 was observed under a microscope, subcultured when the degree of confluence was 80%-90%, and samples were taken after subculture to detect bacteria, fungi, endotoxin and mycoplasma. The test results showed that there was no colony growth after cultured on Columbia blood agar and Sabauro agar plate culture, showing that bacteria and fungi were negative; DNA fluorescent staining method showed no particulate mycoplasma after Hoechst3325 staining, showing that mycoplasma was negative; The test for endotoxin was negative; the above tests were all derived from the pharmacopoeia test methods, and the results showed that they were all negative and met the conditions for reinfusion of biological therapy.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com