Bispiperidine-substituted isoflavone compounds and uses thereof
A technology of compound and composition, applied in the field of isoflavone compounds substituted by bispiperidine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] 1. Preparation of compound I
[0040] Synthesis of 3-(4-methoxyphenyl)-7-(4-bromobutoxy)-4H-chromogen-4-one (2)
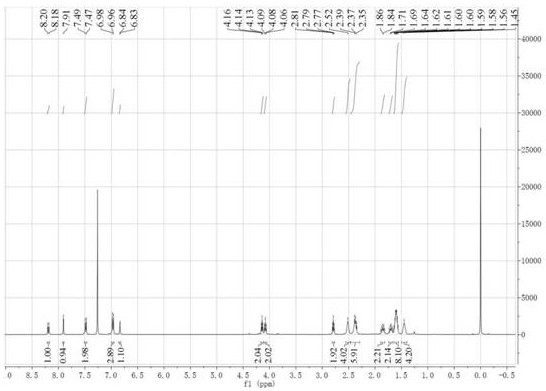
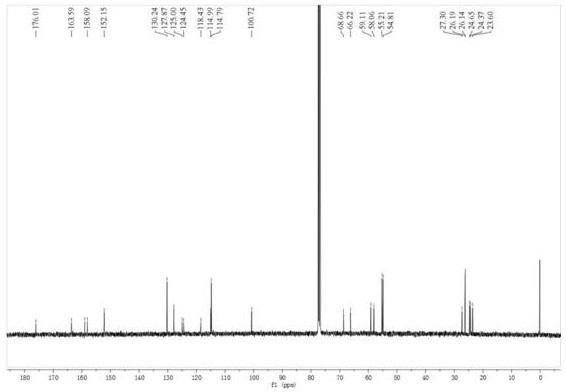
[0041] In a 1000mL single-necked bottle, add formononetin (5g, 18.6mmol), finely ground anhydrous potassium carbonate powder (30g, 217mmol), 500mL acetone, and then add 1,4-dibromobutane (24mL, 198mmol ), heated to reflux for 10 h, concentrated the reaction mixture under reduced pressure, added water to the residue, stirred, filtered with suction, washed the filter cake with water, and dried to obtain 6.62 g of a white solid with a yield of 88.6%. mp: 152.8-154.0°C. 1H NMR (400MHz, CDCl3) δ: 8.21(d, J=8.8Hz, 1H), 7.91(s, 1H), 7.50(d, J=8.8Hz, 2H), 6.99-6.96(m, 3H), 6.84 (d, J=2.4Hz, 1H), 4.10(t, J=6.0Hz, 2H), 3.84(s, 3H), 3.51(t, J=6.0Hz, 2H), 2.14-1.98(m, 4H) ; 13C NMR (100MHz, CDCl3) δ: 175.8, 163.2, 159.6, 157.9, 152.0, 130.1, 127.8, 124.9, 124.2, 118.5, 114.7, 114.0, 100.6, 67.6, 55.3, 33.2, 29.3, 27.6.ESI-MS ( m / z): 403.31 [M+H]+.
[0042] Synthesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com