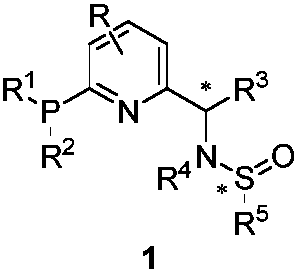
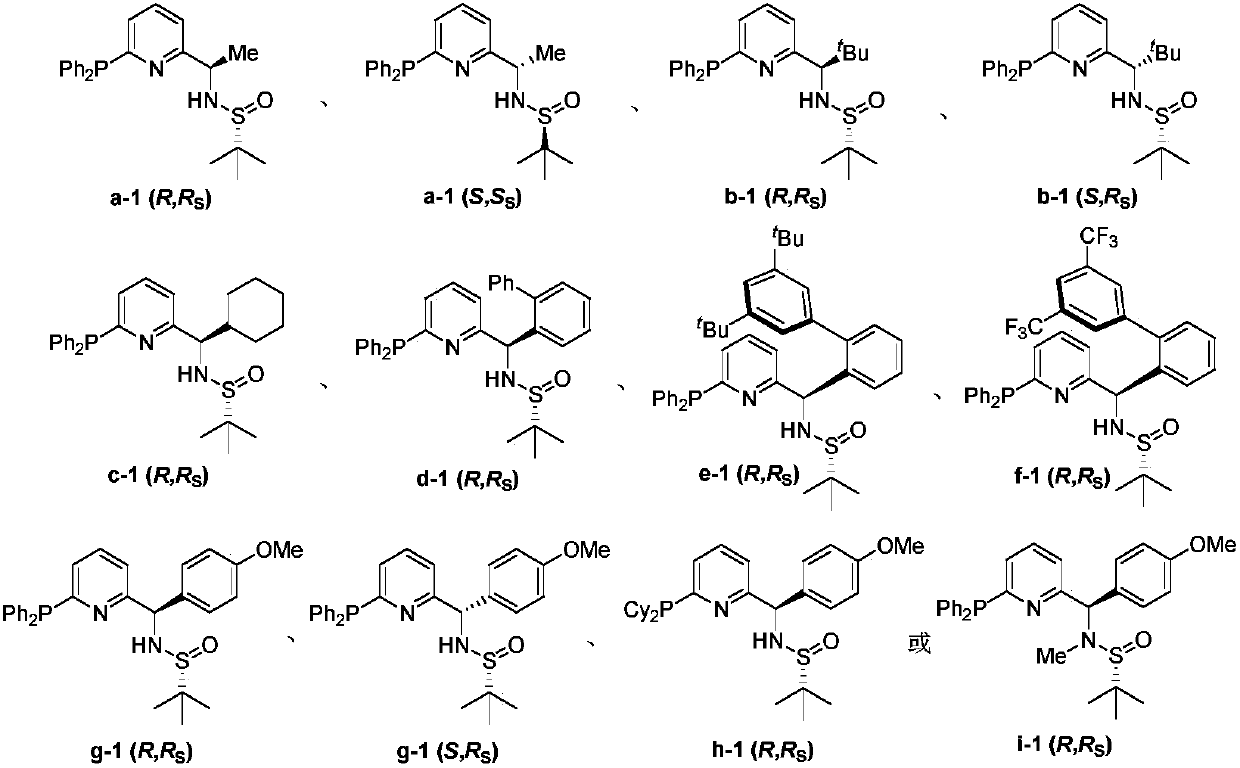
Chiral monophosphine ligand CF-Phos of pyridine skeleton, and preparation method and application thereof
A cf-phos and pyridine technology, which is applied to the new chiral monophosphine ligand CF-Phos modified by pyridine skeleton and its preparation field, can solve the problems of expensive raw materials, complex synthesis routes, hazardous reagents and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
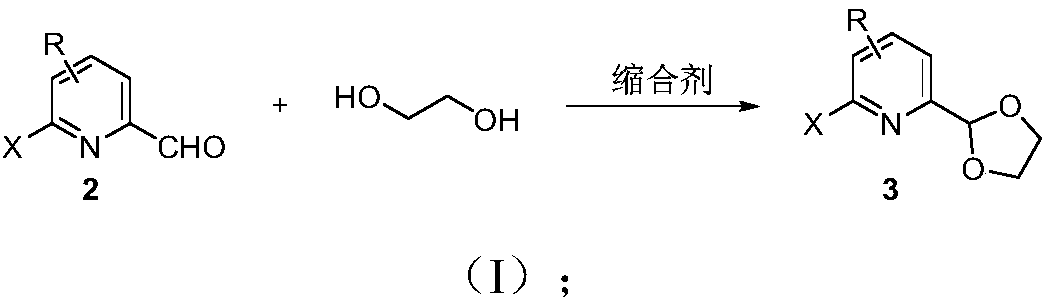
[0108] Example 1 The synthesis (reference scheme one)
[0109] Step 1: In a 250mL single-necked flask, add (20mmol, 3.72g), ethylene glycol (3.0eq., 3.72g), TsOH (0.1eq., 0.38g) and 50mL Toluene, install a water separator, heat and reflux at 150°C for 5 hours, and slowly cool to room temperature Liquid separation, the aqueous layer was extracted three times with ethyl acetate, the organic phases were combined, washed with water and saturated sodium chloride respectively, dried over anhydrous sodium sulfate, filtered, spin-dried, and purified by column chromatography to obtain The yield was 95%.
[0110] Wherein, TsOH is p-toluenesulfonic acid, and Toluene is toluene.
[0111] The second step: the prepared in the first step (10mmol, 2.3g) was added to a 100mL dry reaction eggplant-shaped flask, under argon protection, 30mL THF was added, and KPPh was added at -50°C 2 (1.2eq., 24mL, 0.5 M inTHF), after stirring for 1 hour, the temperature was raised naturally, stirred o...
Embodiment 2
[0118] Example 2 The synthesis (reference scheme one)
[0119] The specific operation is the same as in Example 1, and the raw materials used are replaced by The yield was 76%. 1 H NMR (300MHz, CDCl 3 )δ7.52–7.43(m,1H),7.42–7.30(m,10H),7.10(d,J=76.0Hz,1H),7.02(d,J=9.0Hz,1H),4.92(s,1H ), 4.56(d, J=6.0Hz, 1H), 1.40(d, J=9.0Hz, 3H), 1.30(s, 9H); 31 P NMR (122MHz, CDCl 3 )δ-3.09; 13 C NMR (101MHz, CDCl 3 ), 23.32, 21.64. HRMS (ESI) calculated for [C 23 h 28 N 2 OPS][M+H] + :411.1655; found: 411.1656.
Embodiment 3
[0120] Example 3 The synthesis (reference scheme one)
[0121] The specific operation was the same as in Example 1, except that the metal reagent used was changed to tert-butylmagnesium bromide (4eq., 4mL, 1M inTHF), and the yield was 63%. 1 H NMR (400MHz, CDCl 3 )δ7.41(td, J=8.0,2.4Hz,1H),7.38–7.20(m,10H),7.04(dd,J=8.0,2.0Hz,1H),6.98(d,J=8.0Hz,1H ),5.07(d,J=8.0Hz,1H),3.93(d,J=8.0Hz,1H),1.03(s,9H),0.72(s,9H); 31 P NMR (122MHz, CDCl 3 )δ-2.70; 13 C NMR (101MHz, CDCl 3 )δ161.62,159.76(d,J=8.1Hz),136.57(d,J=9.1Hz),135.96(d,J=9.1Hz),135.29(d,J=5.12Hz),134.90,134.70,134.10,133.91 , 129.09, 128.76–128.29(m), 127.22, 126.95, 122.52, 68.32, 56.00, 36.47, 26.58, 22.99. HRMS (ESI) calculated for [C 26 h 34 N 2 OPS][M+H] + :453.2124; found: 453.2116.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com