Preparation method for L-muscone
A muscone and imine technology is applied in the direction of organic chemical methods, chemical instruments and methods, and preparation of imino compounds. It can solve the problems of cumbersome synthesis of chiral diols, high catalyst costs, and low overall yields. The effect of low cost, high total yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
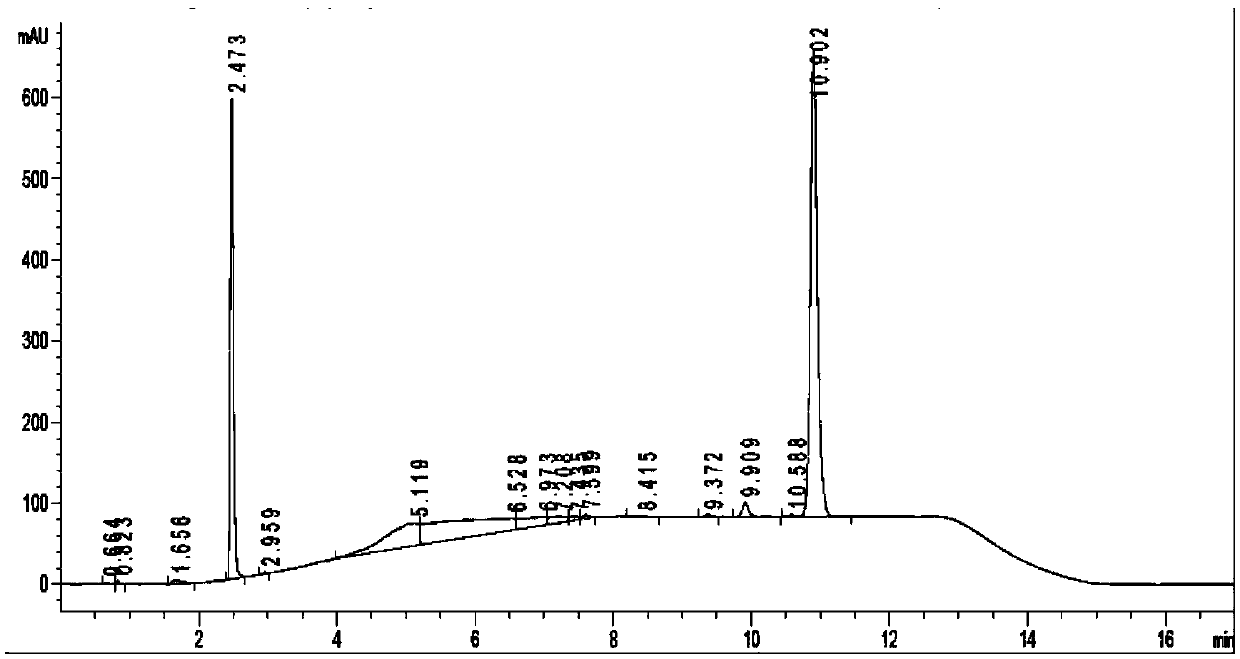
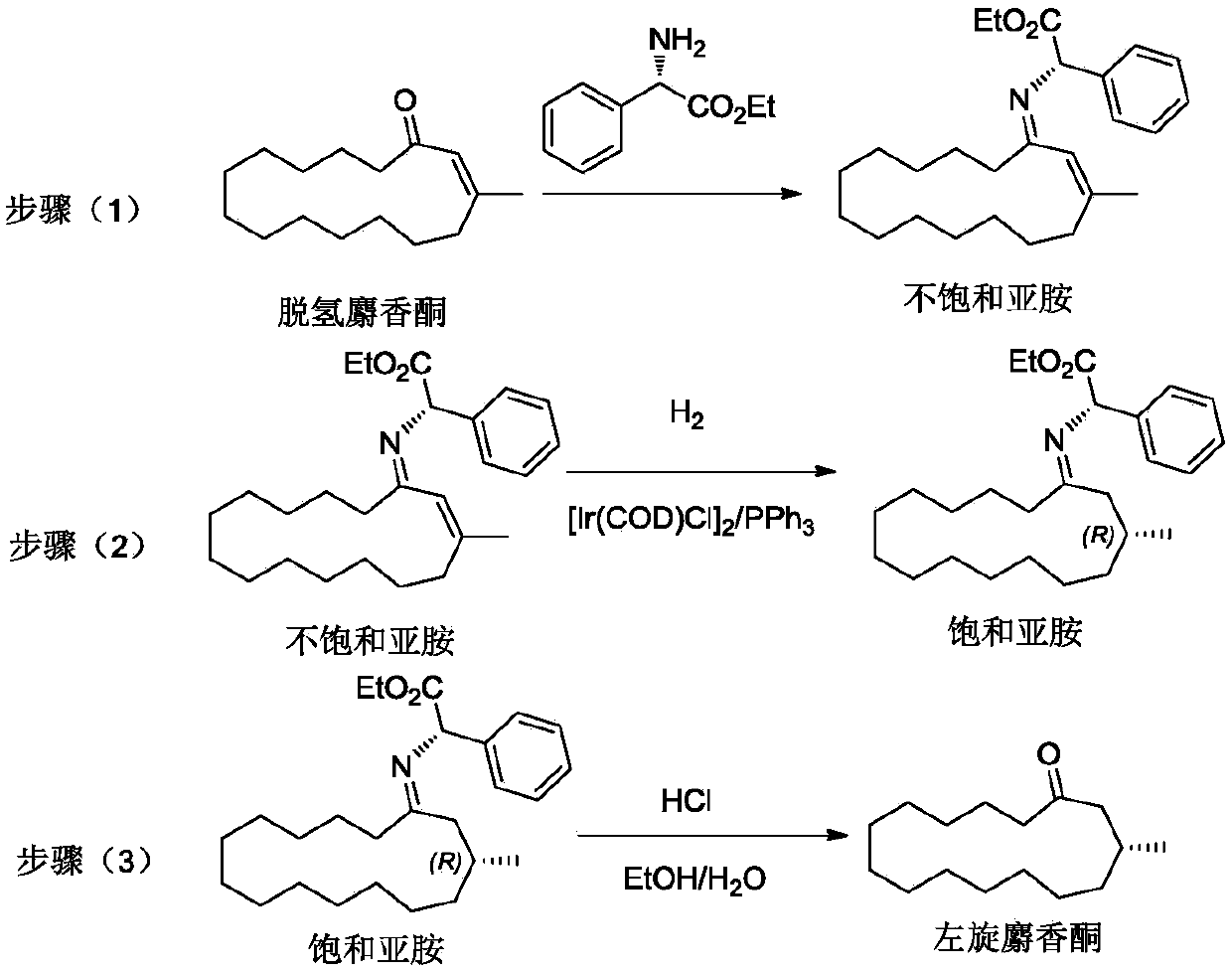
[0047] In the air, in a 2000mL round bottom flask equipped with a condenser, add 118.15g dehydromusketone (500.0mmol), 89.60g (500.0mmol), 4.305g p-toluenesulfonic acid (25mmol) and ethyl acetate (1000.0mL), after nitrogen replacement for 20 minutes, react at 80°C for 4h under nitrogen protection. The reaction solution was cooled to room temperature and washed with Na 2 CO 3 Saturated solution (1000.0mL) and NaCl saturated solution (1000.00mL) were washed twice respectively, anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated with a rotary evaporator, and the liquid phase analysis gave 192.92 g of unsaturated imine, with a yield of 97.07%.
[0048] In the glove box, in a 500mL stainless steel autoclave, add 99.37g of unsaturated imine (250mmol), [Ir(COD)Cl] 2 (0.1mol%, relative to unsaturated imine), triphenylphosphine (100.0mol%, relative to iridium metal precursor) and ethanol (200mL) to seal the autoclave, out of the glove box, and replace nitrogen with hydr...
Embodiment 2
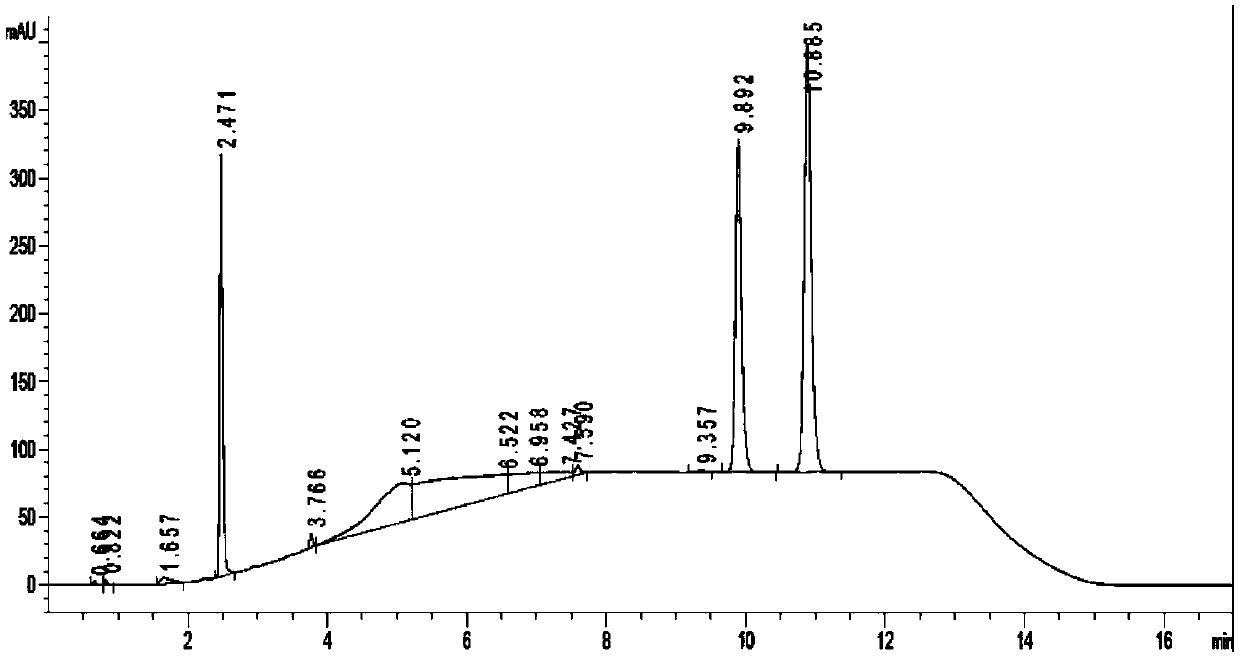
[0055] In the air, in a 2000mL round bottom flask equipped with a condenser, add 118.15g dehydromusketone (500.0mmol), 72.71g (600mmol), 0.05g hydrochloric acid (HCl 37wt%, 0.5mmol) and toluene (100.0mL), after nitrogen replacement for 20 minutes, react at 100°C for 8h under nitrogen protection. The reaction solution was cooled to room temperature and washed with Na 2 CO 3 Saturated solution (1000.0mL) and NaCl saturated solution (1000.00mL) were washed twice respectively, anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated with a rotary evaporator, and the liquid phase analysis gave 163.14 g of unsaturated imine, with a yield of 96.11%.
[0056] In the glove box, in a 500mL stainless steel autoclave, add 84.87g of unsaturated imine (250mmol), [Ir(acac)(CO) 2 ] (5.0 mol%, relative to the unsaturated imine), 1,2-bis(diphenylphosphino)methane (120.0 mol%, relative to the iridium metal precursor) and tetrahydrofuran (200 mL) The autoclave was sealed, and the glove...
Embodiment 3
[0059] In the air, in a 2000mL round bottom flask equipped with a condenser, add 118.15g dehydromusketone (500.0mmol), 59.70g (400.0mmol), 9.8g of sulfuric acid (100mmol) and acetonitrile (250.0mL), after nitrogen replacement for 20 minutes, react at 40°C for 1h under nitrogen protection. The reaction solution was cooled to room temperature and washed with Na 2 CO 3 Saturated solution (1000.0mL) and NaCl saturated solution (1000.00mL) were washed twice respectively, anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated with a rotary evaporator, and the liquid phase analysis gave 137.02 g of unsaturated imine, with a yield of 93.2%.
[0060] In the glove box, in a 500mL stainless steel autoclave, add 91.89g of unsaturated imine (250mmol), [Ir(COD)OMe] 2 (0.5mol%, relative to the unsaturated imine), 1,2-bis(diphenylphosphino)benzene (150.0mol%, relative to the iridium metal precursor) and methanol (200mL) to seal the autoclave, out of the glove box , replace nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com