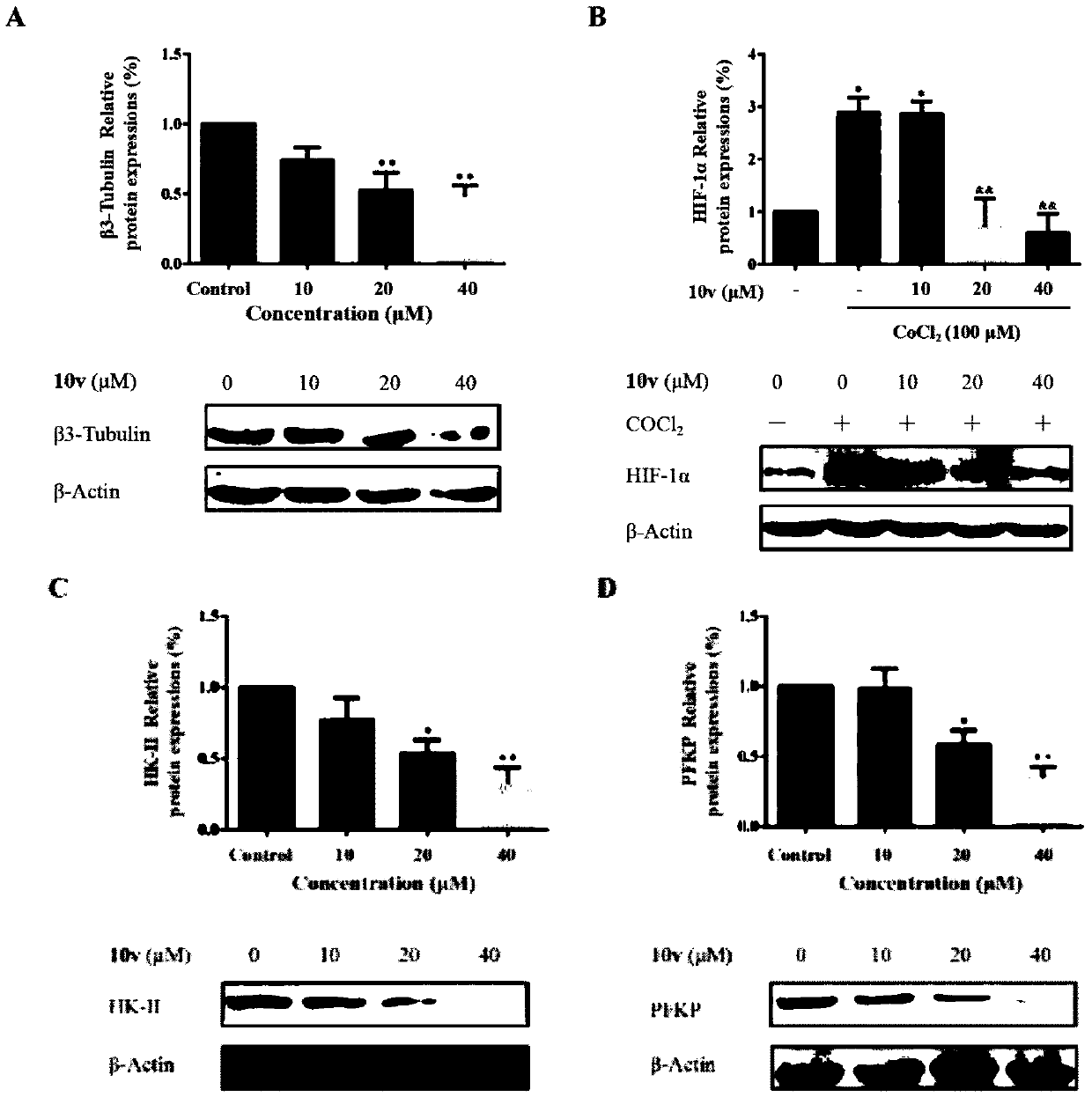
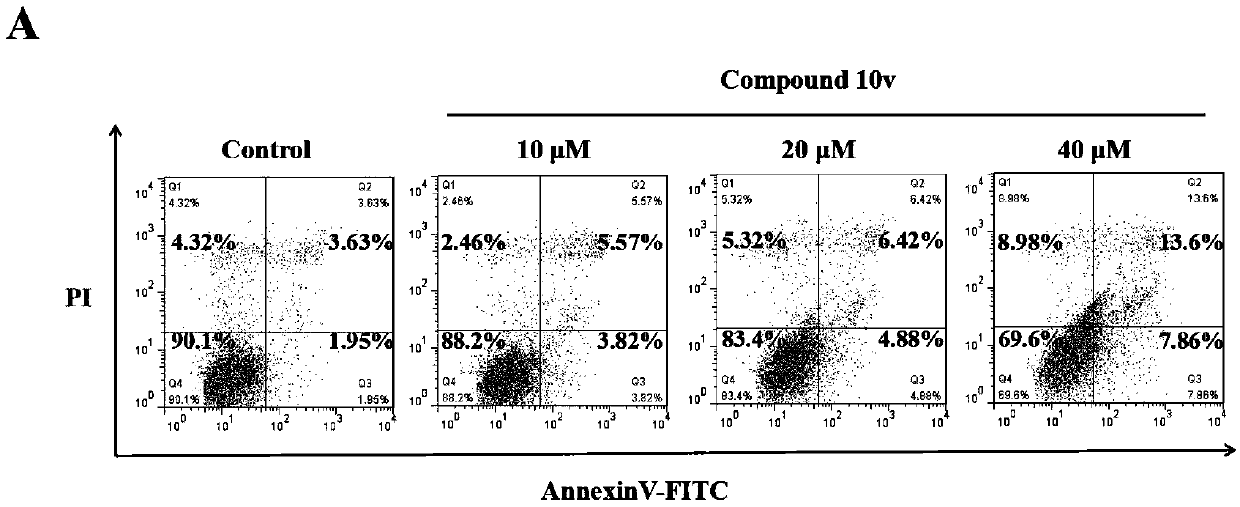
Trimethoxyflavone salicylic acid derivative and antitumor activity thereof
A technology of trimethoxyl and salicylate, which is applied in the direction of antineoplastic drugs, organic active ingredients, drug combinations, etc., can solve the problems of low curative effect and side effects of chemotherapy drugs, so as to accelerate death, block nutrient supply, broaden the range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0038] Example 13', 4', 5'-trimethoxy-7-hydroxyflavone synthesis
[0039]
[0040] Synthesis of 3'4'5-trimethoxy-7-hydroxyflavone (3): Place resorcinol (2.5 g, 0.023 mol) in a 100 mL three-neck flask, add 30 mL of anhydrous ether, and cool in an ice bath , after the raw materials are completely dissolved, add chloroacetonitrile (2 mL, 0.032 mol) and a catalytic amount of anhydrous zinc chloride, mix well, introduce dry hydrogen chloride, after 3 hours, place the reaction flask at 4 °C for 1 d, and then pass hydrogen chloride for 2 hours at 4 °C Set aside for 1 d, filter, and wash with anhydrous ether to obtain an orange-yellow precipitate (i.e., intermediate 2). Dissolve the washed orange-yellow precipitate in a round-bottomed flask with an appropriate amount of hot water, heat under reflux in a water bath for 1 h, stand at 4°C overnight, and pump Filtration to obtain compound 2-chloro-1-(2,4-dihydroxyphenyl)ethanone (3).
[0041] Compound 2-chloro-1-(2,4-dihydroxyphenyl)e...
Embodiment 2
[0042] Example 2 Synthesis of 7-(4-bromobutoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one
[0043]
[0044] Add (4.63g, 0.010mol) 3',4',5'-trimethoxy-7-hydroxyflavone (4), anhydrous potassium carbonate and 100ml acetone to a 250mL round-bottomed flask in turn, heat and stir to reflux, and then gradually add (2.59 g, 0.012 mol) 1,4-dibromobutane was added dropwise, heated at 60°C and condensed to reflux, the solution became clear and then turbid; the reaction progress was detected by thin-layer chromatography, and the intermediate 8 was obtained after column chromatography purification. The reagent is: methanol: dichloromethane=1:50-150:1; the yield is about 52%.
Embodiment 3
[0045] Example 3 Synthesis of methyl 5-chloro-2-hydroxybenzoate (9)
[0046]
[0047] Dissolve 5-chloro-2-hydroxybenzoic acid (1.73 g, 0.010 mol) and its derivatives in 30 mL of methanol, add a catalytic amount of thionyl chloride under ice bath conditions, and heat and stir at 60 °C for 48 h; thin layer chromatography Method to detect the reaction progress, after the reaction is complete, the methanol is evaporated to dryness under reduced pressure, dissolved in dichloromethane, extracted with aqueous sodium carbonate solution, and then dichloromethane is evaporated to dryness under reduced pressure to obtain methyl 5-chloro-2-hydroxybenzoate (9 ) with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com