Method for refining caprolactam
A technology of caprolactam and refining method, which is applied in the field of chemical production, can solve the problems of short regeneration period and easy saturation of ion exchange resin, and achieve the effects of reducing waste water discharge, reducing operating load and prolonging life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
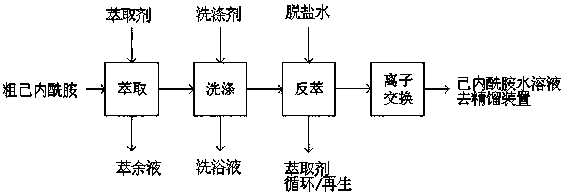
[0020] Cyclohexanone oxime and niacin are subjected to Beckmann rearrangement reaction to obtain rearrangement liquid. The rearrangement liquid is subjected to hydrolysis, ammonia causticization, and phase separation, and the crude caprolactam with about 4% ammonium sulfate and about 25% water is obtained from the light phase. The crude caprolactam was extracted with benzene to obtain benzene caprolactam. Styrene liquid index: conductivity, 80μs / cm; extinction value 1.04; caprolactam concentration 19%. The washing solution used in the washing process is 27.5% mass concentration of hydrogen peroxide solution, the addition is 2g / kg benzene ethyl alcohol, the washing temperature is 35 degrees, the washing equipment is a washing tower, and the tower form is a large-pore sieve plate tower. The conductivity of the washed benzene ethyl alcohol solution was 10 μs / cm, and the extinction value was 0.16. Use desalted water to back-extract the washed benzene-hexane solution. The back-ex...
Embodiment 2
[0022] Cyclohexanone oxime and niacin are subjected to Beckmann rearrangement reaction to obtain rearrangement liquid. The rearrangement liquid is subjected to hydrolysis, ammonia causticization, and phase separation, and the crude caprolactam with about 4% ammonium sulfate and about 25% water is obtained from the light phase. The crude caprolactam was extracted with benzene to obtain benzene caprolactam. Styrene liquid index: conductivity, 80μs / cm; extinction value 1.04; caprolactam concentration 19%. The washing solution used in the washing process is 50% mass concentration of hydrogen peroxide solution, the addition amount is 10g / kg benzene ethyl alcohol, the washing temperature is 40 degrees, and the washing equipment is a static mixer and a phase separation tank. The electrical conductivity of the washed benzene ethyl alcohol solution is 8 μs / cm, and the extinction value is 0.10. Use desalted water to back-extract the washed benzene-hexane solution. The back-extraction ...
Embodiment 3
[0024] Cyclohexanone oxime and niacin are subjected to Beckmann rearrangement reaction to obtain rearrangement liquid. The rearrangement liquid is subjected to hydrolysis, ammonia causticization, and phase separation, and the crude caprolactam with about 4% ammonium sulfate and about 25% water is obtained from the light phase. The crude caprolactam was extracted with benzene to obtain benzene caprolactam. Styrene liquid index: conductivity, 80μs / cm; extinction value 1.04; caprolactam concentration 19%. The washing solution used in the washing process is 10% mass concentration of hydrogen peroxide solution, the addition is 1g / kg benzene ethyl alcohol, the washing temperature is 50 degrees, the washing equipment is a washing tower, and the tower form is a large-pore sieve plate tower. The conductivity of the washed benzene ethyl alcohol solution was 9 μs / cm, and the extinction value was 0.15. Use desalted water to back-extract the washed benzene-hexane solution. The back-extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com