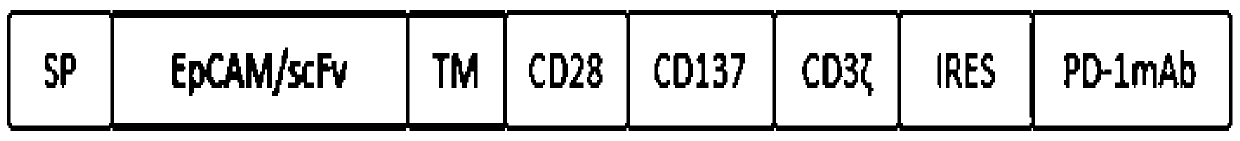
Preparation and construction method of CAR-T carrier applied in colon cancer treatment
A technology of colon cancer and carrier, which is applied in the field of biomedicine to achieve the effects of strong proliferation, enhanced killing effect, and enhanced immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Construction of therapeutic vectors for CAR-T preparation
[0044] (1) Plasmid extraction:
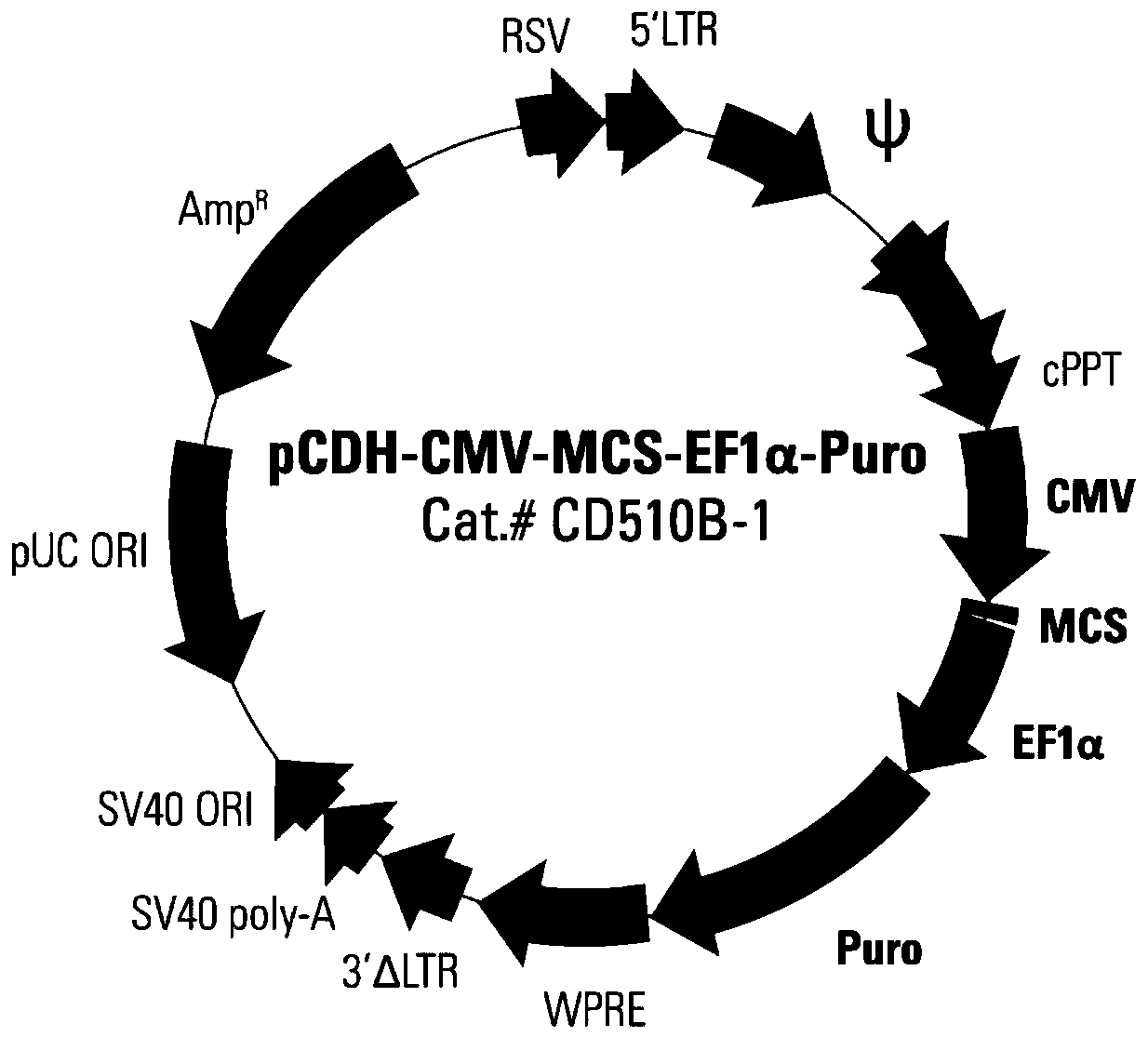
[0045] Take out the glycerol bacteria containing the lentiviral expression plasmid pCDH-CMV-MCS-EF1-Puro and PUC19 plasmid from the -80°C refrigerator respectively, take 5 μL and inoculate them into 5 mL LB liquid medium (AMP resistance), shake on a constant temperature shaker Cultivate for 12-16 hours at 37°C and 250rpm.
[0046] Plasmid extraction was carried out according to the instructions of Tiangen's plasmid mini-extraction kit.
[0047] (2) Digestion, ligation, transformation
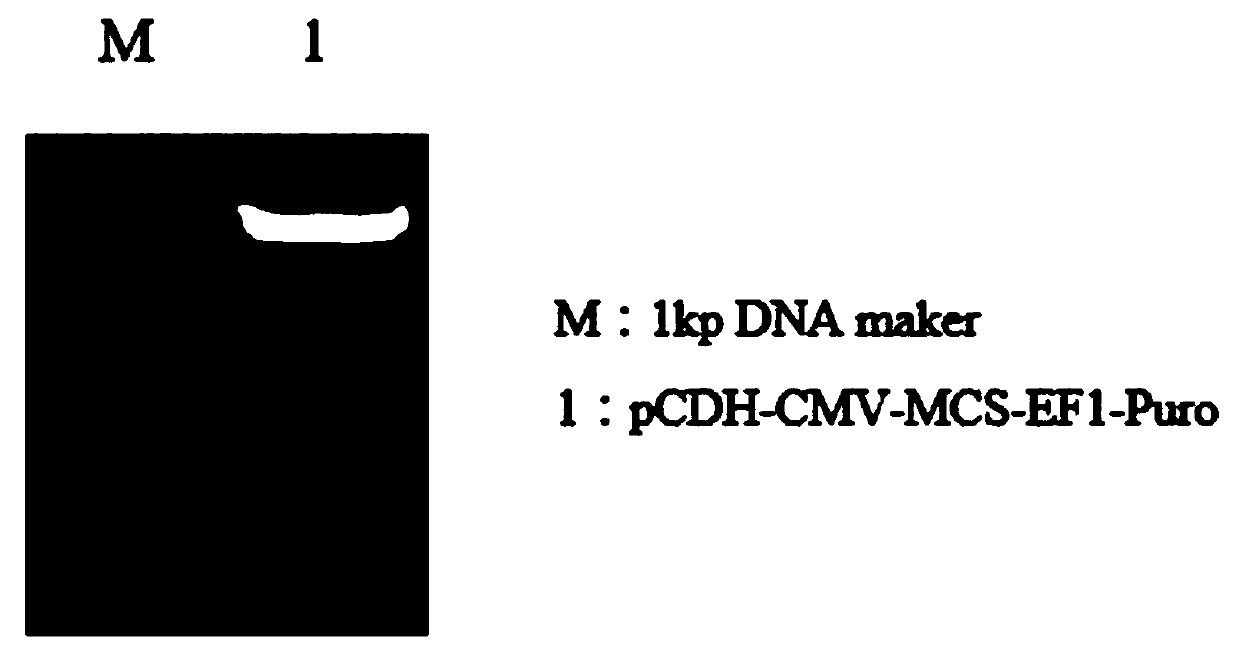
[0048] The extracted lentiviral expression plasmids pCDH-CMV-MCS-EF1-Puro and PUC19 plasmids were simultaneously digested with EcoRI and NotI, and the digested products were subjected to agarose gel electrophoresis, and the results were observed and recorded with a gel imager , pCDH-CMV-MCS-EF1-Puro agarose gel electrophoresis results are as follows image 3 shown.
[0049] Use the...
Embodiment 2
[0063] Embodiment 2: the preparation of concentrated virus liquid
[0064] The three-plasmid packaging system was used for lentiviral packaging. The three plasmids are respectively the therapeutic vector plasmid obtained in Example 1, the lentiviral packaging plasmid pMD2G and the vector plasmid PSPAX2, and the cells are 293T cells.
[0065] The specific implementation steps are as follows:
[0066] (1) Plating within 24 hours before transfection: select cells with a passage number of less than 3 generations, adjust the cell density according to the cell growth density and state, and select 293T cells with a growth density of 80% for plating. In this example, a 6 cm dish was selected. ;
[0067] (2) When the cell growth density reaches 60-90% and the cells are in good condition, the virus packaging can be carried out. The virus packaging is carried out according to the ratio of lentivirus packaging mixture and vector plasmid = 3:2, and the required three-plasmid mixture rati...
Embodiment 3
[0078] Example 3: Acquisition of CAR-T cells
[0079] 1. PBMC (peripheral blood mononuclear cell) isolation
[0080] (1) Use a vacuum blood collection tube containing heparin to collect about 6mL of human peripheral fresh blood;
[0081] (2) Dilution: add an equal volume of PBS at room temperature, and gently pipette to mix;
[0082] (3) Adding samples: Take a 50mL centrifuge tube, draw 6mL Ficoll (lymphocyte separation solution) into the centrifuge tube (the volume ratio of Ficoll to blood before dilution is 1:1), tilt the tube at 45°, and put the diluted blood in About 1cm above the Ficoll liquid level, slowly add to the Ficoll along the tube wall;
[0083] (4) Centrifugation: 18-20°C, 2000rpm, 30min. After centrifugation, it is divided into four layers from the bottom of the tube to the liquid surface, which are red blood cell and granulocyte layer, stratified liquid layer, mononuclear cell layer, and plasma layer;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com