Iguratimod intermediate and synthesis method thereof
A synthesis method and a technology for intermediates, applied in the field of chemical drug synthesis, can solve the problems of low purity, excessively long reaction steps, low yield and the like, and achieve the effects of simplified reaction steps, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
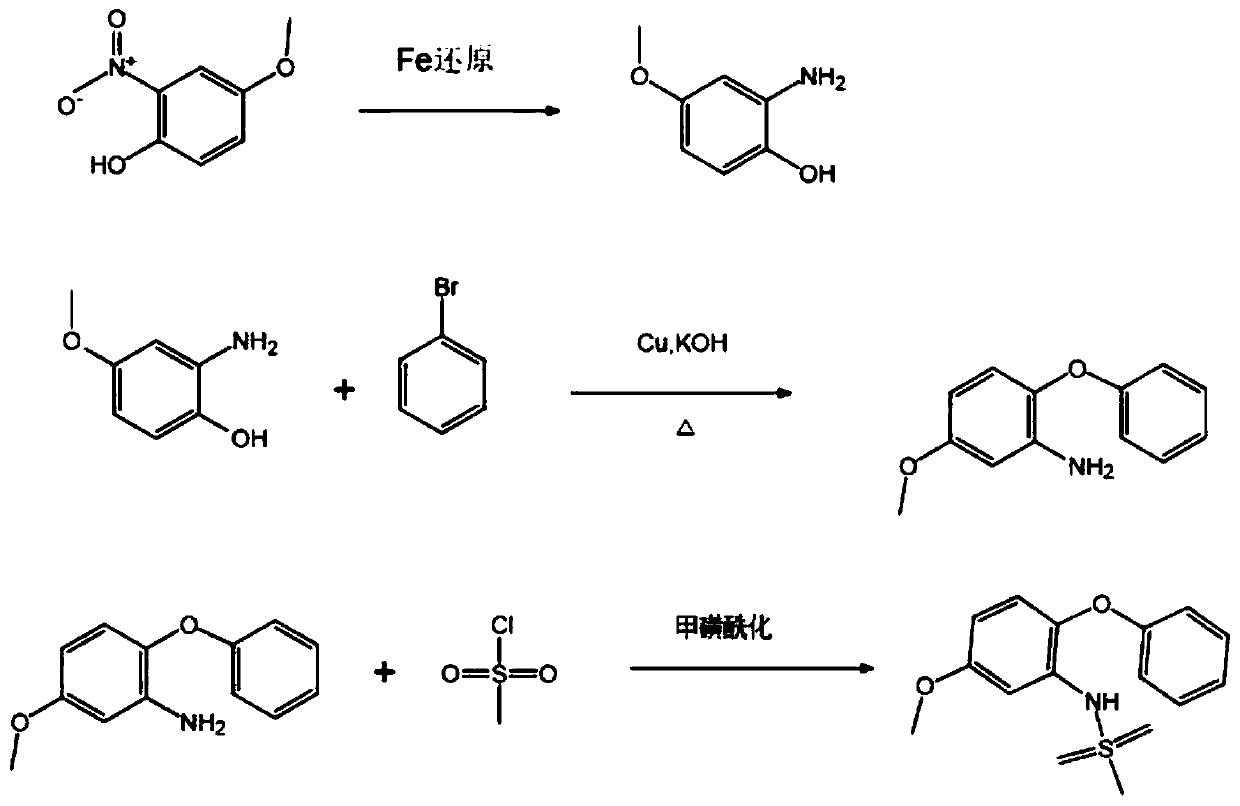
[0024] Such as figure 1 As shown, this embodiment 1 provides a synthetic method of the key intermediate of Alamod, including:
[0025] Step S1, reduction, that is, using 4-methoxy-2 nitrophenol to prepare 2-amino-4-methoxyphenol;
[0026] Step S2, Ullmann reaction, that is, the preparation of 3-amino-4-phenoxy-anisole through 2-amino-4-methoxyphenol; and
[0027] Step S3, mesylation, that is, preparing 5-methoxy-2-phenoxy-benzenesulfonamide through 3-amino-4-phenoxy-anisole.
[0028] As an optional implementation of reduction.
[0029] The reduction method includes: dissolving 4-methoxy-2 nitrophenol in a mixed solution of 70% ethanol and 4N HCl, raising the temperature of the reaction solution to 65-75°C; then adding iron powder evenly in three batches within 20 minutes into the reaction solution; stir the reaction quickly, and control until there is no raw material; after the reaction is completed, add a saturated solution of sodium carbonate to adjust the pH to 8-9 to ob...
Embodiment 2
[0040] On the basis of Example 1, this Example 2 provides a key intermediate of iguratimod, which is suitable for being prepared by the aforementioned synthesis method.
[0041] For the composition and content of the key intermediates of iguratimod and the specific implementation process, please refer to the relevant discussion in Example 1, which will not be repeated here.
Embodiment 3
[0043] (1) Dissolve 169g of 4-methoxy-2 nitrophenol in a mixed solution of 1500ml 70% ethanol and 40ml 4N HCl, and heat up the reaction solution to 65°C; add 140g iron powder evenly in three batches within 20min In the reaction solution, the reaction was stirred rapidly, and controlled until there was no raw material; after the reaction was completed, a saturated solution of sodium carbonate was added to adjust the pH to 8 to obtain 2-amino-4-methoxyphenol.
[0044] (2) Add 67.2g of powdered potassium hydroxide to the 2-amino-4-methoxyphenol prepared in (1), stir, and heat at 150°C for 2.5 hours, until the reaction is complete to obtain dry salt; Add 0.63g of copper powder and 157g of bromobenzene in sequence, stir at 160°C until the color changes, then raise the temperature to 190°C, and heat for 1.5 hours. After cooling, pour 1200ml of water and 300ml of ether, and distill to obtain 3-amino-4-phenoxy-anisole.
[0045](3) Dissolve the 3-amino-4-phenoxy-anisole prepared in (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com