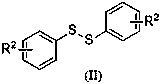
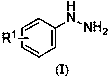
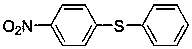Diaryl sulfide compounds and preparation method thereof
A technology of diaryl sulfide and diaryl disulfide, which is applied in the field of preparation of diaryl sulfide compounds, can solve problems such as long time, high cost, and danger of aryl diazonium compounds, and meet the reaction conditions Mild, easy to operate, safe and economical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation method of diaryl sulfide compound, the steps are as follows:
[0023] In a 25 mL reaction tube, add photosensitizer Eosin Y disodium salt (5 mol%), hydrogen peroxide (1 equiv), solvent dimethyl sulfoxide 1.5 mL, phenylhydrazine 0.5 mmol, diphenyl disulfide 4.0 mmol, stirring in the air to control the reaction temperature to 45 °C, and reacting under blue light for 8 hours, the final product was separated by silica gel column chromatography, and the yield of the final product was 68% based on the molar amount of phenylhydrazine as 100%.
[0024] The specific results are as follows:
[0025]
[0026] 1 H NMR (400 MHz, CDCl 3 ): δ 7.48 – 7.43 (m, 4H), 7.42 – 7.36 (m, 4H), 7.36– 7.30 (m, 2H). 13 C NMR (101 MHz, CDCl 3 ): δ 135.90, 131.15, 129.32, 127.16.
Embodiment 2
[0028] The preparation method of diaryl sulfide compound, the steps are as follows:
[0029] Add photosensitizer Eosin Y disodium salt (5 mol%), hydrogen peroxide (1 equiv), solvent dimethyl sulfoxide 1.5 mL, 4-methoxyphenylhydrazine 0.5 mmol, diphenyl 2.0 mmol of yl disulfide, stirred in the air to control the reaction temperature to 25 °C, reacted under blue light irradiation for 8 hours, and separated by silica gel column chromatography to obtain the final product.
[0030] The specific results are as follows:
[0031]
[0032] 1 H NMR (400 MHz, CDCl 3 ) δ 7.50 (d, J = 2.1 Hz, 1H), 7.48 (d, J = 2.2 Hz,1H), 7.33 – 7.27 (m, 2H), 7.26 – 7.18 (m, 3H), 6.97 (d, J = 2.2 Hz, 1H), 6.96(d, J = 2.1 Hz, 1H), 3.87 (s, 3H). 13 C NMR (101 MHz, CDCl 3 ): δ 159.91, 138.73, 135.49, 129.02, 128.23, 125.83, 124.31, 115.07, 55.42.
Embodiment 3
[0034] The preparation method of diaryl sulfide compound, the steps are as follows:
[0035] Add photosensitizer Eosin Y disodium salt (5 mol%), hydrogen peroxide (1 equiv), solvent dimethyl sulfoxide 1.5 mL, 4-nitrophenylhydrazine 0.5 mmol, diphenyl 2.0 mmol of disulfide was stirred in the air to control the reaction temperature to 35 °C, reacted under blue light irradiation for 8 hours, and separated by silica gel column chromatography to obtain the final product.
[0036] The specific results are as follows:
[0037]
[0038] 1 H NMR (400 MHz, CDCl 3 ): δ 8.26 (dd, J = 8.3, 1.5 Hz, 1H), 7.66 – 7.59(m, 2H), 7.52 (dd, J = 5.0, 1.9 Hz, 3H), 7.36 (m, 1H), 7.24 (m ,1H), 6.89 (dd,J = 8.2, 1.3 Hz, 1H). 13 C NMR (101 MHz, CDCl 3 ): δ145.00, 139.50, 135.92, 133.41, 131.02, 130.12, 130.02, 128.32, 125.76, 124.94.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com