1,2-di(pyridyl)-3,4-di(4-pyridylvinyl-3-fluorobenzene)cyclobutane and preparation method therefor
A technology based on pyridyl vinyl and pyridyl, which is applied in 1 field to achieve the effects of mild reaction conditions, simple and easy-to-operate process, and excellent fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
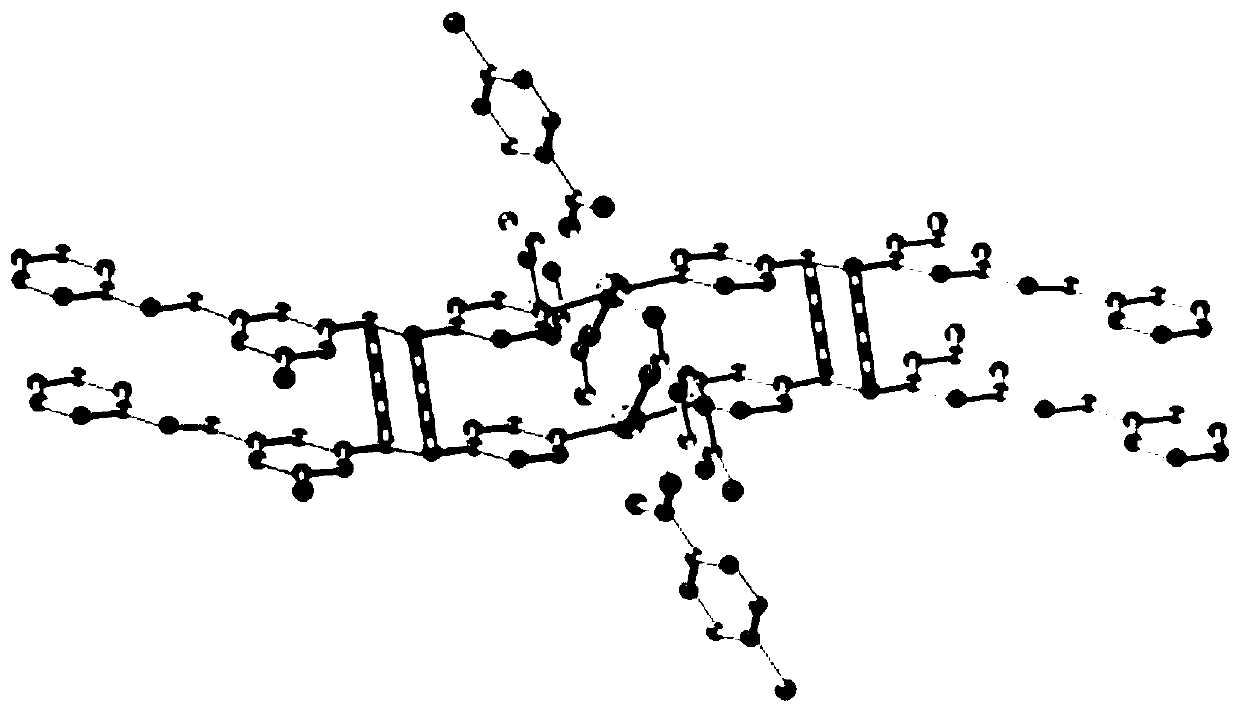
[0025] 1) Take 30.2 mg (0.1 mmol) of 3,5-bis(2-pyridine vinyl) fluorobenzene, Cd(NO 3 ) 2 4H 2 O30.8mg (0.1mmol) and p-chlorobenzoic acid 15.6mg (0.1mmol) join in the rigid glass tube of one end closure, add 4mL water in the glass tube, adjust the pH= of the system with nitric acid (concentration is 65%) 3. Melt and seal the other end of the glass tube, and then place the glass tube at 100°C for 30 hours of constant temperature reaction. After the reaction is completed, lower it to room temperature (the cooling rate is 5°C / h), take it out, and you can see that the inner wall of the glass tube A pale yellow crystal was precipitated, which was intermediate 1 (120.8 mg, yield 78%).
[0026] The characterization of intermediate 1 is as follows:
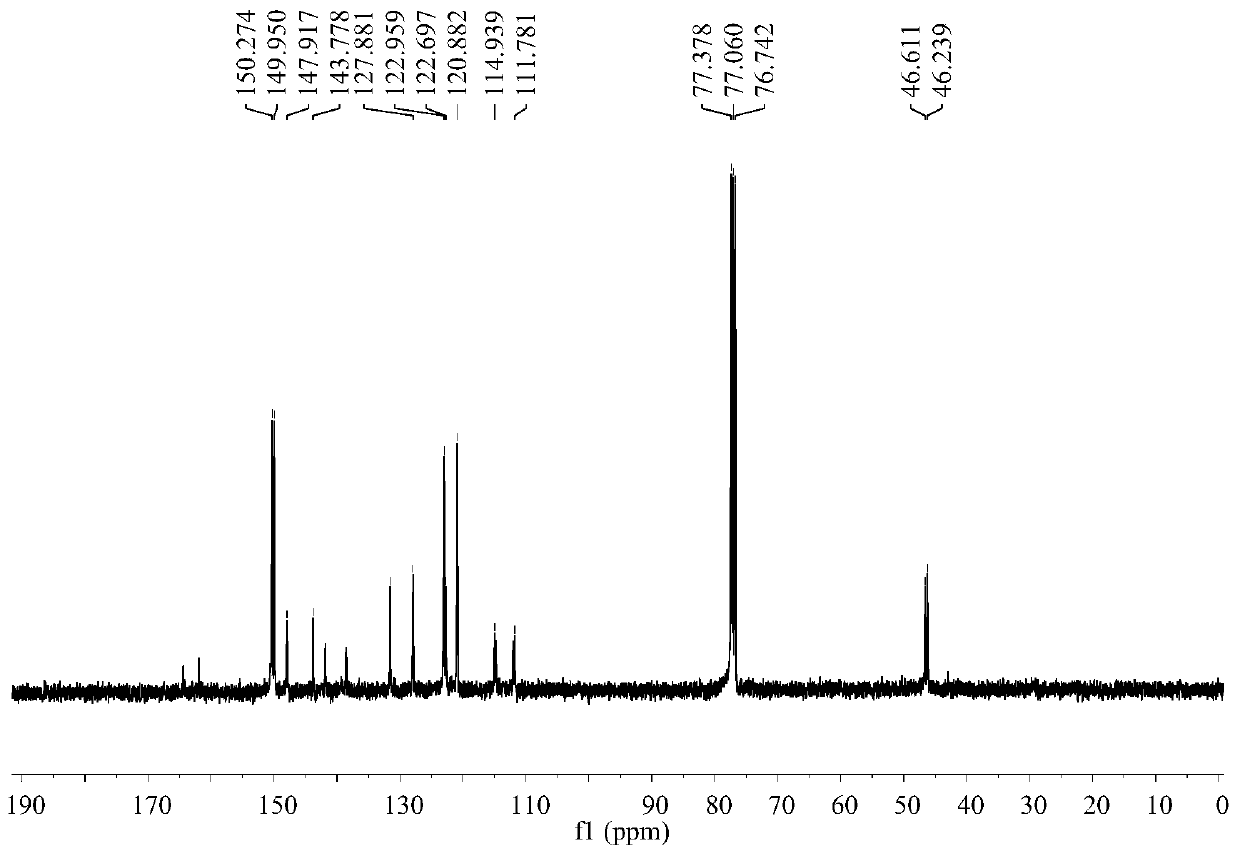
[0027] IR:v(KBr) / cm -1 3071(w), 1614(s), 1585(s), 1532(s), 1392(s), 1269(m), 1215(m), 1165(w), 1089(w), 1014(m), 969(m), 877(m), 845(m), 770(m), 663(m), 534(m), 439(m);
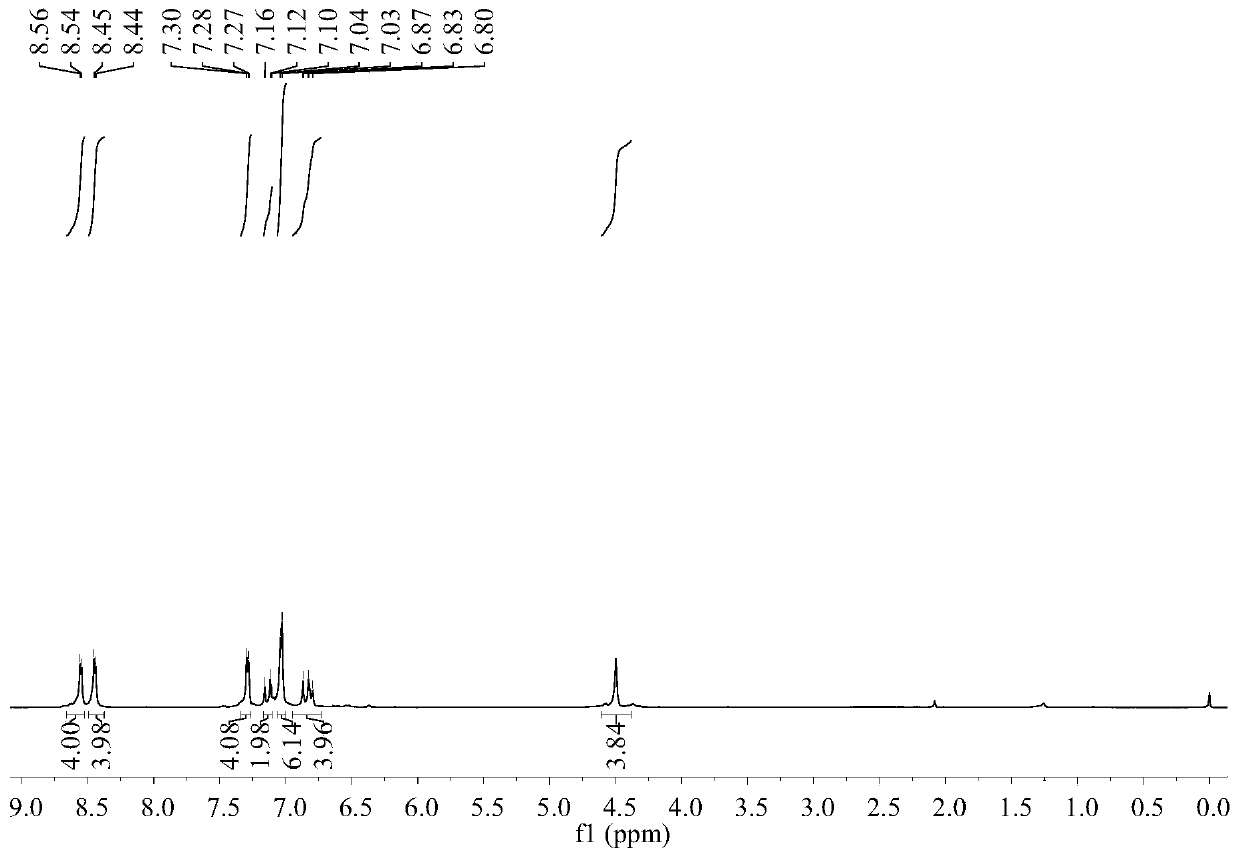
[0028] 1 H NMR (400MHz, d 6 -DMSO, 298K, TMS): δ=8.62 (d, ...
Embodiment 2
[0060] 1) Take 30.2 mg (0.1 mmol) of 3,5-bis(2-pyridine vinyl) fluorobenzene, Cd(NO 3 ) 2 4H 2 O30.8mg (0.1mmol) and p-chlorobenzoic acid 15.6mg (0.1mmol) join in the rigid glass tube of one end closure, add 2mL water in the glass tube, adjust the pH of the system with nitric acid (concentration is 69%)= 4. Melt and seal the other end of the glass tube, and then place the glass tube at 80°C for 36 hours of constant temperature reaction. After the reaction is completed, lower it to room temperature (the cooling rate is 10°C / h), take it out, and you can see the inner wall of the glass tube Bright yellow crystals were precipitated, which were intermediate 1 (108.4 mg, yield 70%);
[0061] 2) Take the obtained single crystal of Intermediate 1, grind it to 200-300 meshes, and then irradiate the ground powder under an ultraviolet lamp with an emission wavelength of 254nm for 4 hours (it can be observed that the color of the powder becomes darker during the irradiation process) , ...
Embodiment 3
[0065] 1) Take 30.2 mg (0.1 mmol) of 3,5-bis(2-pyridine vinyl) fluorobenzene, Cd(NO 3 ) 2 4H 2 O30.8mg (0.1mmol) and p-chlorobenzoic acid 15.6mg (0.1mmol) join in the rigid glass tube of one end closure, add 5mL water in the glass tube, adjust the pH= of system with hydrochloric acid (concentration is 36%) 1. Melt and seal the other end of the glass tube, then place the glass tube at 120°C for a constant temperature reaction for 12 hours. After the reaction is completed, lower it to room temperature (the cooling rate is 8°C / h), take it out, and you can see the inner wall of the glass tube Bright yellow crystals were precipitated, which were intermediate 1 (92.9 mg, yield 60%);
[0066] 2) Take the obtained single crystal of intermediate 1, grind it to 200-300 mesh, and then irradiate the ground powder under an ultraviolet lamp with an emission wavelength of 350nm for 3 hours (it can be observed that the color of the powder becomes darker during the irradiation process) , to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com