Method for measuring robenidine hydrochloride and metabolite residues in aquatic product through high performance liquid chromatography-tandem mass spectrometry
A technology of high performance liquid chromatography and chlorpheniramine hydrochloride, which is applied in measuring devices, instruments, scientific instruments, etc., to achieve the effects of low detection limit, simple operation, high accuracy and precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Method optimization for the determination of prohezidine hydrochloride and its metabolite residues in aquatic products by high performance liquid chromatography-tandem mass spectrometry
[0027] 1. Optimization of mass spectrometry conditions
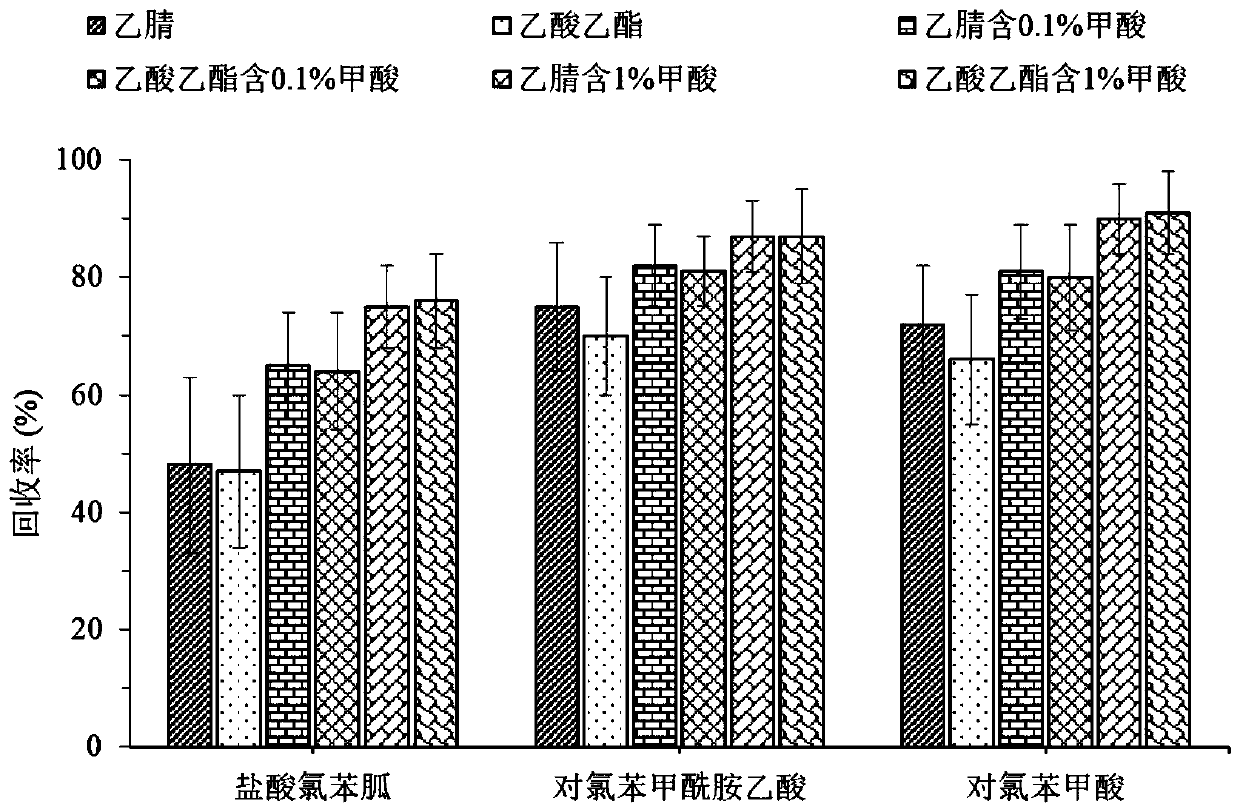
[0028] Inject 10 mg / L of chlorhexenidine hydrochloride, chlorhexidine hydrochloride-d8, p-chlorobenzamide acetic acid, and p-chlorobenzoic acid into the mass spectrometer through a syringe pump, and the responses of the above-mentioned target substances in positive ion and negative ion modes The results showed that the responsivity of chlorhexidine hydrochloride and chlorhexidine-d8 in positive ion mode was 5 times and 3 times that in negative ion mode. However, the response values of p-chlorobenzamide acetic acid and p-chlorobenzoic acid in the negative ion mode were 2 times and 1 times that in the positive ion mode. Therefore, chlorhexidine hydrochloride and chlorhexidine-d 8 The analysis was performed in positive...
Embodiment 2
[0037] A method for the determination of prohezidine hydrochloride and its metabolite residues in aquatic products based on high performance liquid chromatography-tandem mass spectrometry of time-segmented technology, the steps are:
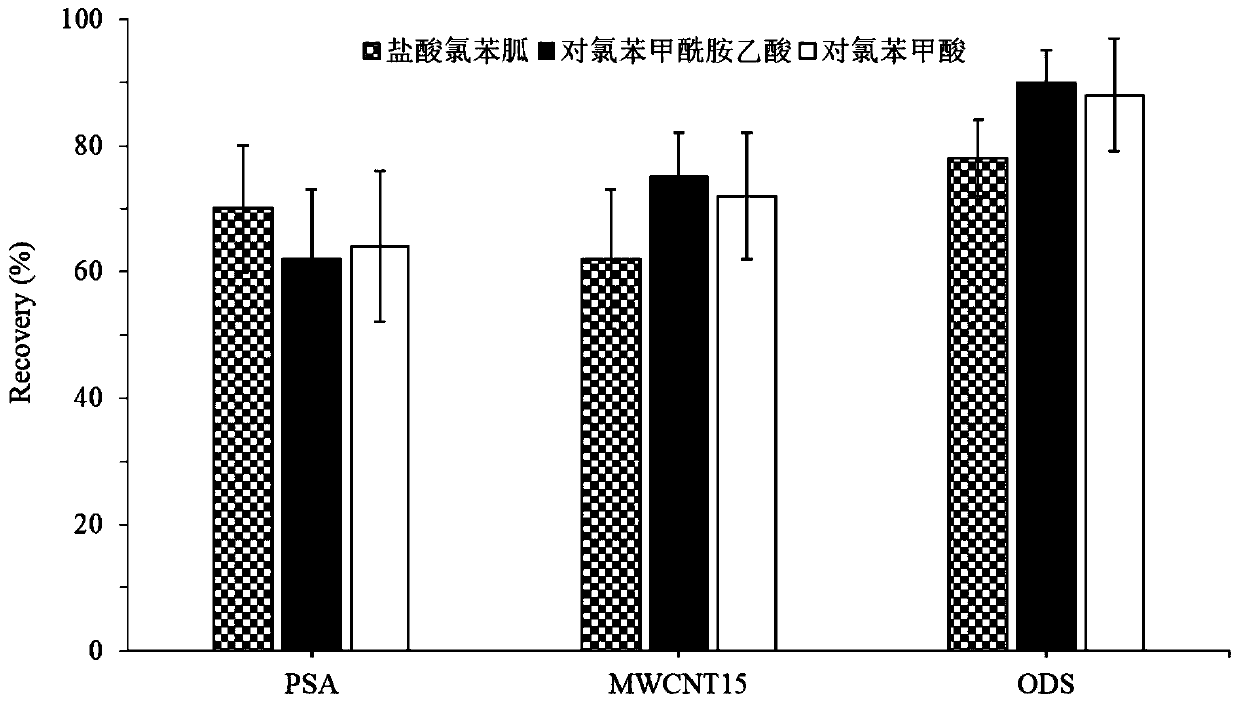
[0038] A. Weigh 1g of homogeneous grass carp muscle sample in a 15mL centrifuge tube, add chlorhexenidine hydrochloride, p-chlorobenzamide acetic acid and p-chlorobenzoic acid standard solution to make chlorhexenidine hydrochloride, p-chlorobenzamide The concentrations of acetic acid and p-chlorobenzoic acid were 1, 3, 50, 100, 400 μg / kg, 2.5, 5, 50, 100, 400 μg / kg, 5, 10, 50, 50 μg / kg, and then 20 μL of 1 mg / kg L Chlorhexidine Hydrochloride-d 8 Internal standard solution, shake for 30s, add 5mL extractant acetonitrile containing 1% formic acid, shake for 30s, ultrasonically extract for 5min, add 1g of anhydrous magnesium sulfate to remove water and part of fat in the sample, vortex. Centrifuge at 8000r / min for 5min, put the upper liquid in anot...
Embodiment 3
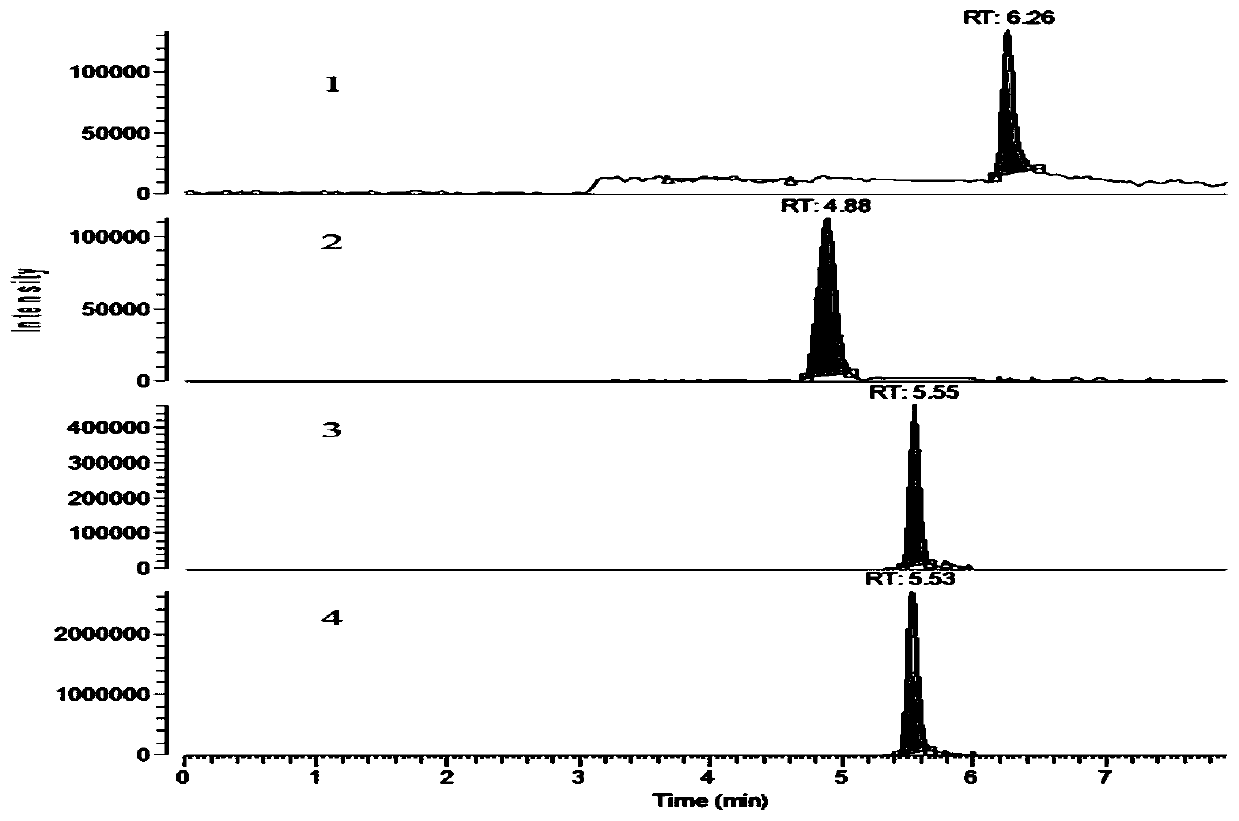
[0056] Application of time-segmented high performance liquid chromatography-tandem mass spectrometry for the determination of prohezidine hydrochloride and its metabolite residues in aquatic products:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com