Duck reovirus vaccine and preparation method thereof
A reovirus and vaccine technology, which is applied in the field of duck reovirus vaccine and its preparation, can solve the problems of economic loss in the duck industry, achieve stable quality, good immune effect, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
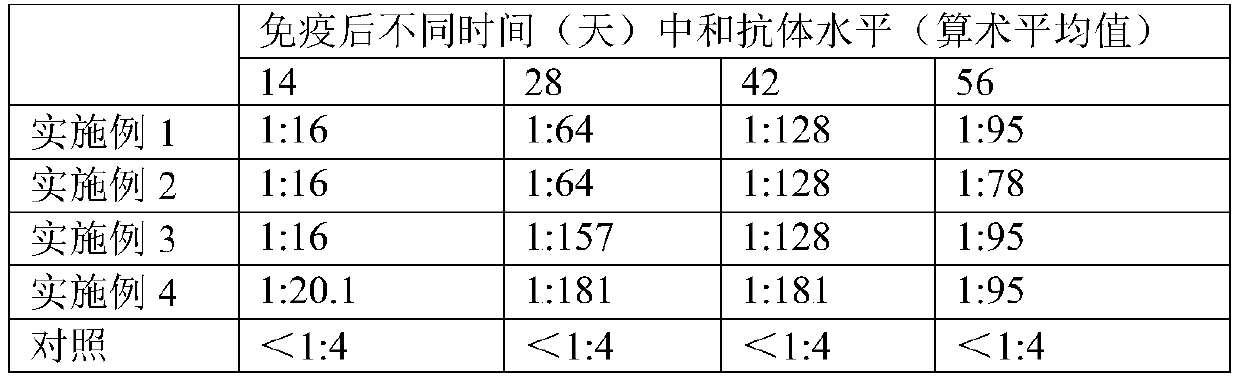
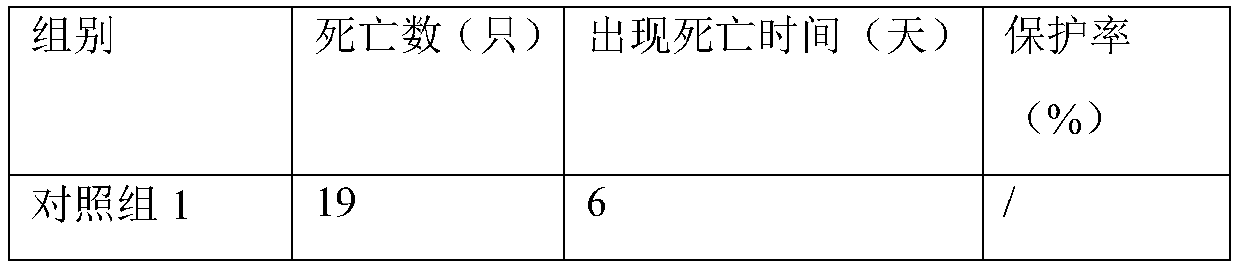
Embodiment 1
[0025] Preparation:
[0026] (1) Digest and disperse BHK-21 cells with trypsin, add 5% newborn bovine serum in DMEM culture solution in a spinner bottle at 37°C, 5% CO 2 After culturing to a monolayer of cells, the original culture medium was discarded, and the cells were washed 3 times with serum-free DMEM medium for duck reovirus inoculation.
[0027] (2) Virus inoculation and cultivation: Inoculate the duck reovirus seed into the cells prepared in step ① according to the final volume of 1:100, and absorb the virus solution at 37°C for 30 minutes. DMEM culture solution of bovine serum at 37°C, 5% CO 2 Culture until the cells are terminated when lesions appear.
[0028] (3) Virus collection, concentration and purification and determination of virus content: the collected cell virus liquid was repeatedly frozen and thawed twice at -20°C, centrifuged at 4°C and 5000rpm for 10min, and the supernatant after centrifugation was used for ultrafiltration and concentration . After...
Embodiment 2
[0031] Preparation:
[0032] (1) Digest and disperse the BHK-21 cells with trypsin, add 10% newborn calf serum to the DMEM culture solution in a microcarrier reactor at 37°C and 5% CO 2 After culturing to a single layer of cells, the original culture medium was discarded, and the cells were washed 3 times with serum-free DMEM medium for duck reovirus inoculation.
[0033] (2) Virus inoculation and cultivation: inoculate the duck reovirus seed into the cells prepared in step ① according to the final volume of 1:1000, and absorb the virus liquid after 60 minutes at 37°C, and then use 2% newborn bovine Serum in DMEM medium at 37°C, 5% CO 2 Culture until the cells are terminated when lesions appear.
[0034] (3) Virus collection, concentration and purification: freeze-thaw the above-mentioned inoculated diseased cells, collect the supernatant after centrifugation to obtain the virus stock solution; concentrate the above-mentioned virus solution by ultrafiltration to obtain the s...
Embodiment 3
[0037] Preparation:
[0038] (1) Digest and disperse the BHK-21 cells with trypsin, add 7% newborn bovine serum to the DMEM culture solution in a microcarrier reactor at 37°C and 5% CO 2 After culturing to a single layer of cells, the original culture medium was discarded, and the cells were washed 3 times with serum-free DMEM culture medium containing 2 mM glutamine for duck reovirus inoculation.
[0039] (2) Virus inoculation and cultivation: Inoculate the duck reovirus seed into the cells prepared in step ① according to the final volume of 1:500, and absorb the virus liquid after 45 minutes at 37°C, and use 1.5% newborn bovine Serum in DMEM medium at 37°C, 5% CO 2 Culture until the cells are terminated when lesions appear.
[0040] (3) Virus collection, concentration and purification: freeze-thaw the above-mentioned inoculated diseased cells, collect the supernatant after centrifugation to obtain the virus stock solution; concentrate the above-mentioned virus solution by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com