Isocyanate monomer preparation method and system
An isocyanate and monomer technology, applied in the field of phosgenation reaction to prepare isocyanate, can solve the problems of long process flow, many side reactions and high energy consumption, and achieve the effects of simple process, stable product quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
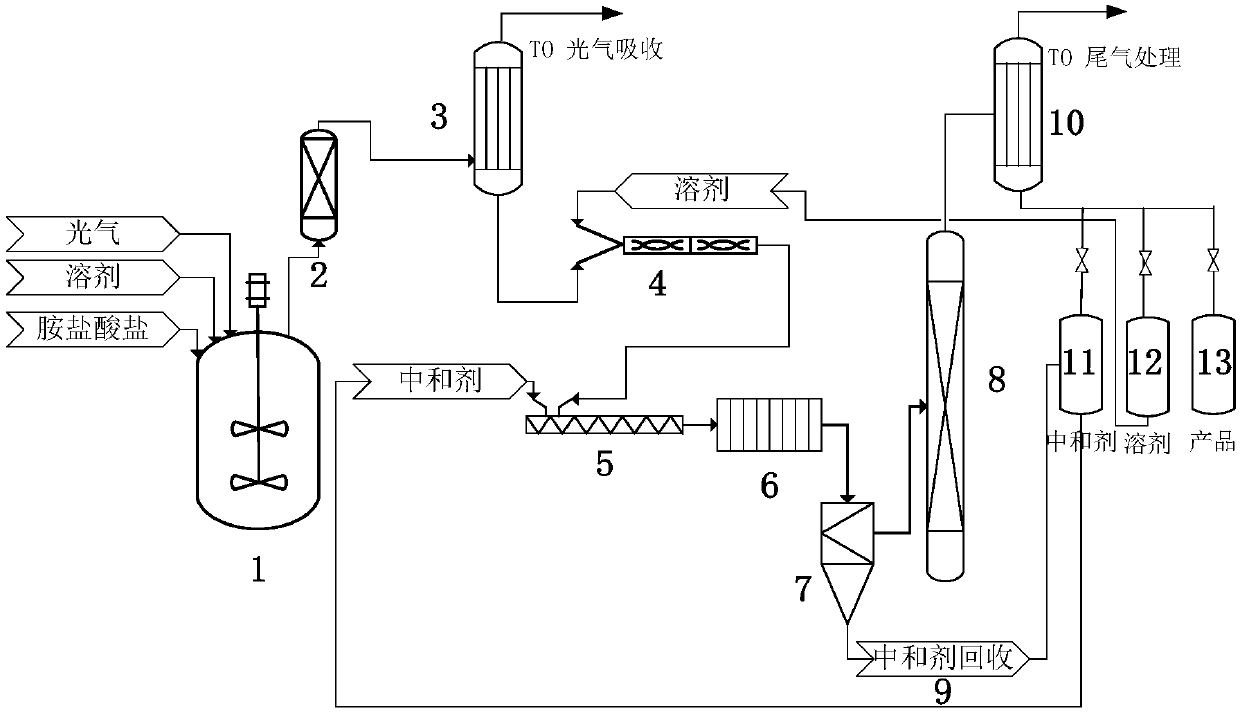
[0066] Using the above process steps, add toluene in the normal pressure reactor, stir and heat up after nitrogen replacement, use toluene as solvent, introduce methylamine hydrochloride and phosgene at a molar ratio of 1:10, 4kgf / cm 2The reaction is carried out at 110°C. After the reaction, the reaction product including carbamoyl chloride, phosgene, and HCl is heated to 75-80°C. The reaction product enters the gas-liquid separator, and then passes through the reaction gas phase condenser. Use 10-25 The chilled water at ℃ controls the temperature of the condenser, and the uncondensed phosgene and hydrogen chloride enter the phosgene absorption treatment process, and the condensed liquid including carbamoyl chloride and isocyanate is exchanged with toluene in a static state after heat exchange in the reaction gas phase condenser to 15-30 ℃ Mix in mixer, methylcarbamoyl chloride (MCC): toluene=1:8 (m / m), triethylamine is neutralizer, methylcarbamoyl chloride (MCC): triethylamine...
Embodiment 2
[0069] Using the above process steps, add o-dichlorobenzene in the normal pressure reactor, stir and heat up after nitrogen replacement, use o-dichlorobenzene as solvent, introduce methylamine hydrochloride and phosgene at a molar ratio of 1:10, 5kgf / cm 2 The reaction is carried out at 110°C and 150°C to generate, and the reaction products obtained after the reaction including ethylcarbamoyl chloride, phosgene, and HCL are heated to 100-105°C and then enter the gas-liquid separator, which is controlled by 15-30°C chilled water The uncondensed phosgene and hydrogen chloride at the temperature of the condenser pass through the reaction gas phase condenser and then enter the phosgene absorption treatment process, including the condensed liquid of ethylcarbamoyl chloride and ethyl isocyanate condensed to 25-35°C, and o-dichlorobenzene Mix in a static mixer, carbamoyl chloride (MCC): o-dichlorobenzene = 1:8 (m / m), triethylamine as neutralizer, carbamoyl chloride (MCC): triethylami...
Embodiment 3
[0071] Afterwards, several synthesis experiments were carried out and tested, and compared with the conventional cleavage reaction, the comparison results are shown in Table 1.
[0072] Table 1. Comparison of indicators of the two processes
[0073] Test items
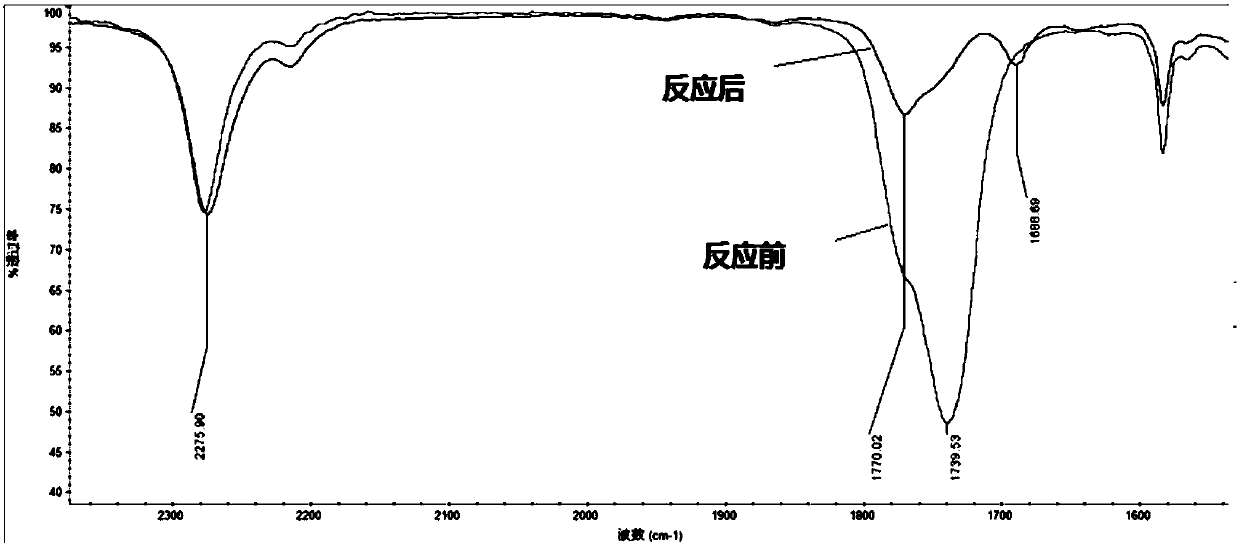
[0074] In the above implementation examples, Agilent 7890 was used to measure the purity and concentration of the reaction solution. IR is tested using FTIR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com