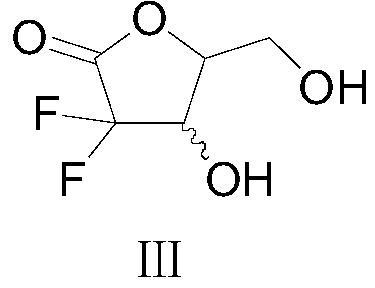
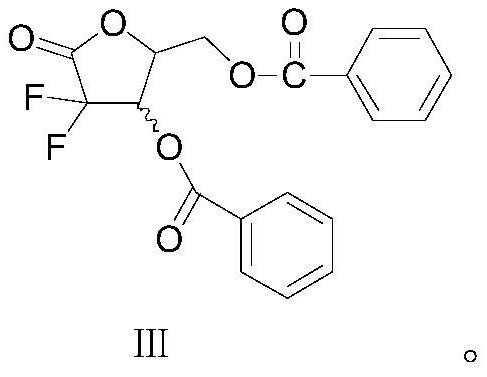
A kind of method utilizing chiral catalyst to prepare gemcitabine intermediate
A chiral catalyst, gemcitabine technology, applied in the direction of organic chemistry, can solve the problems of inability to select threo and erythro, increase the cost of raw and auxiliary materials, and low erythro content, so as to increase the overall reactivity and increase nucleophilicity performance, effect of reducing key order
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The δ-aminoalcohol ligand in the present embodiment is as follows:
[0033]
[0034] Among them, R 1 and R 2 Same, both methyl.
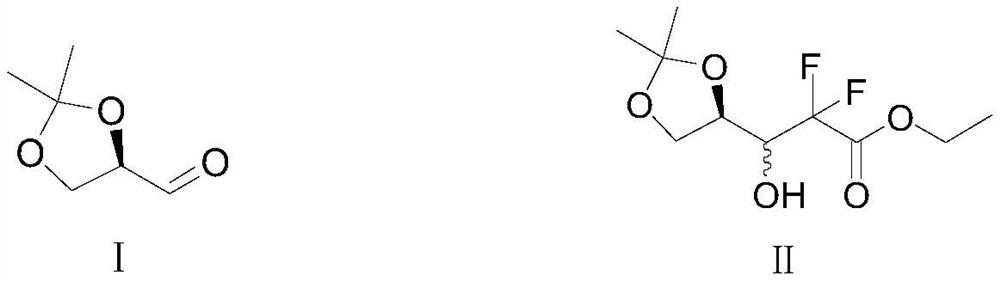
[0035]Add 100g of tetrahydrofuran solvent, 15g of zinc powder and 0.3g of δ-aminoalcohol ligand to the clean reactor, stir and cool down to 0°C, then start to drop R-glyceraldehyde acetone and ethyl difluorobromoacetate Among them, R-glycerol aldehyde acetone 23g, ethyl difluorobromoacetate: 35g; control the temperature at 0-5°C during the dropping process, and continue to control the temperature at 0-5°C after the dropping is completed Insulated condensation reaction for 2 hours, TLC detected the completion of the reaction, after the reaction, dropwise added hydrochloric acid with a concentration of 5% to quench, when the pH value of the reaction solution was adjusted to 5-6, stopped the dropwise addition, and then allowed to stand for stratification. Collect the organic layer, wash it with saturated sodium bicarbonate solution to pH =...
Embodiment 2
[0038] The δ-aminoalcohol ligand in the present embodiment is as follows:
[0039]
[0040] Among them, R 1 and R 2 Same, both methyl.
[0041] Add 120g of tetrahydrofuran solvent, 20g of zinc powder and 0.5g of δ-aminoalcohol ligand to a clean reactor, stir and cool down to 0°C, then start to drop R-glyceraldehyde acetone and ethyl difluorobromoacetate Among them, R-glycerol aldehyde acetone 23g, ethyl difluorobromoacetate: 35g; control the temperature at 0-5°C during the dropwise addition, and continue to control the temperature at -5-0°C after the dropwise addition Carry out the heat preservation condensation reaction for 2 hours, TLC detects that the reaction is complete, after the reaction is completed, dropwise add hydrochloric acid with a concentration of 5% to quench, when the pH value of the reaction solution is adjusted to 5-6, stop the dropwise addition, and then, let it stand for stratification . Collect the organic layer, wash it with saturated sodium bicar...
Embodiment 3
[0044] The δ-aminoalcohol ligand in the present embodiment is as follows:
[0045]
[0046] Among them, R 1 and R 2 Same, both methyl.
[0047] Add 150g of tetrahydrofuran solvent, 22g of zinc powder and 0.9g of δ-aminoalcohol ligand to the clean reactor, stir and cool down to 0°C, then start to dropwise add R-glyceraldehyde acetone and ethyl difluorobromoacetate Among them, R-glycerol aldehyde acetone 23g, ethyl difluorobromoacetate: 35g; control the temperature at 0-5°C during the dropwise addition, and continue to control the temperature at -5-0°C after the dropwise addition The heat preservation condensation reaction was carried out for 2 hours, and the reaction was detected by TLC. After the reaction was completed, sulfuric acid was added dropwise to quench. When the pH value of the reaction solution was adjusted to 5-6, the dropwise addition was stopped, and then the mixture was allowed to stand for stratification. Collect the organic layer, wash it with saturated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com