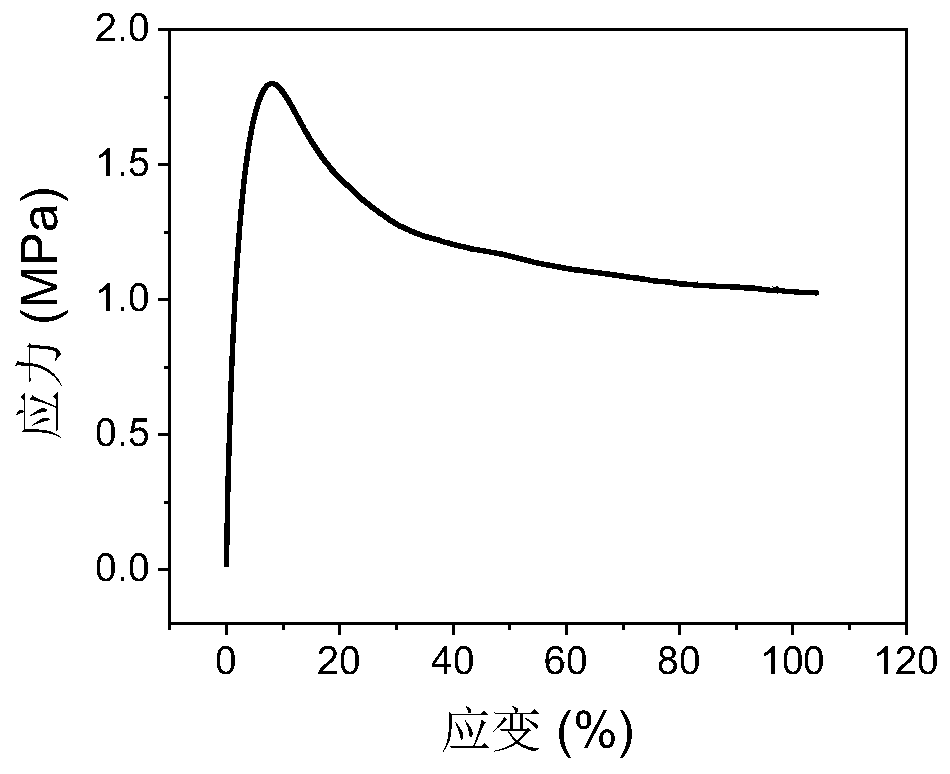
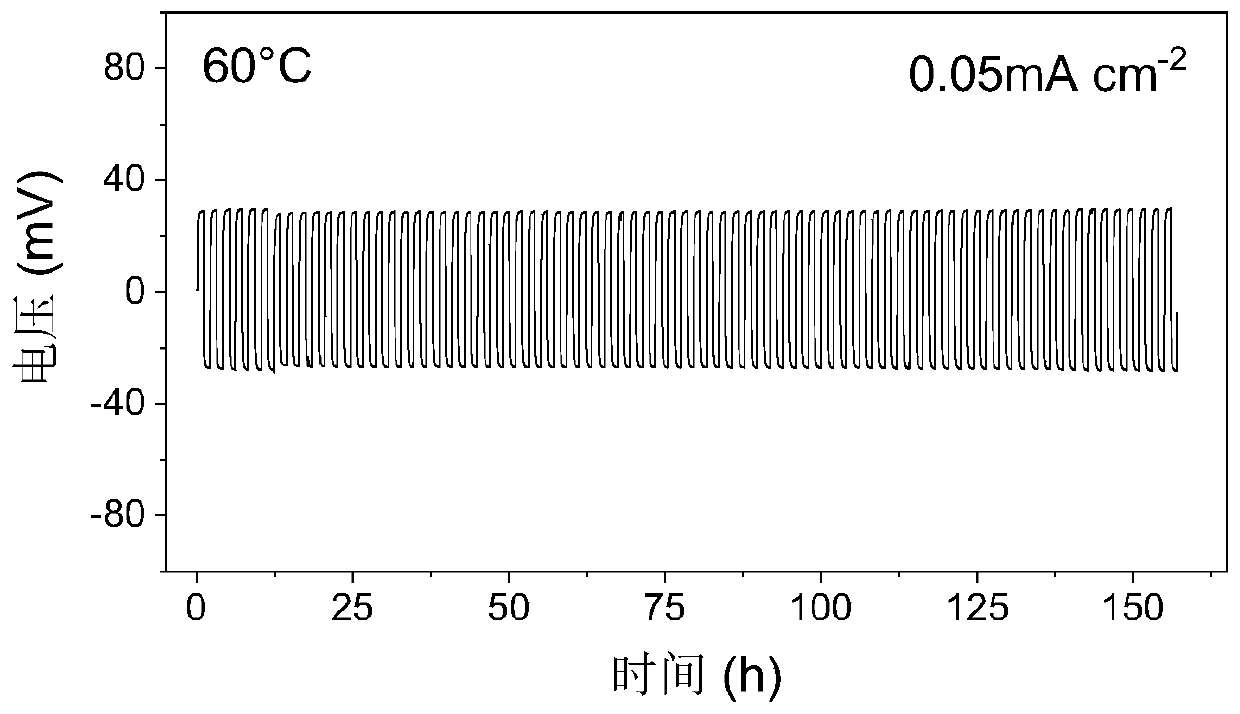
Preparation method of composite solid polymer electrolyte
A solid-state polymer and electrolyte technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of inability to take into account the performance of polymer electrolytes, unfavorable large-scale production, and decline in mechanical properties, and achieve good safety and stability. stability and cycling performance, capacity retention, effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This embodiment includes the following steps:
[0026] (1) Add urea to a quartz crucible, heat at 550 degrees Celsius for 4 hours in an air atmosphere, and obtain graphite phase carbon nitride g-C with a thinner sheet thickness 3 N 4 , where the heating rate is 5°C / min.
[0027] (2) the graphite phase carbon nitride g-C prepared in the step (1) 3 N 4 , polyethylene oxide PEO, and lithium bistrifluoromethanesulfonimide LiTFSI were mixed, and then acetonitrile was added and stirred until the system was uniform to obtain a mixed solution.
[0028] Based on the mass of polyethylene oxide PEO, lithium bistrifluoromethanesulfonimide LiTFSI accounts for 30% of its mass fraction; graphite phase carbon nitride g-C 3 N 4 Accounting for 5% of its mass fraction, the amount of acetonitrile added is such that polyethylene oxide PEO, bistrifluoromethanesulfonimide lithium LiTFSI and graphite phase carbon nitride g-C can be completely dissolved. 3 N 4 A mixture of the three is e...
Embodiment 2
[0040] This embodiment includes the following steps:
[0041] (1) Dicyandiamide is added in a quartz crucible, and heated for 8 hours at 500 degrees Celsius in an air atmosphere to obtain graphite phase carbon nitride g-C with a thinner sheet thickness 3 N 4 , where the heating rate was 5°C / min.
[0042] (2) the graphite phase carbon nitride g-C prepared in the step (1) 3 N 4 , polyethylene oxide PEO, and lithium bistrifluoromethanesulfonimide LiTFSI are mixed, and then acetonitrile is added and stirred until the system is uniform to obtain a mixed solution;
[0043] Based on the mass of polyethylene oxide PEO, lithium bistrifluoromethanesulfonimide LiTFSI accounts for 20% of its mass fraction; graphite phase carbon nitride g-C 3 N 4 The mass fraction is 1%, and the amount of acetonitrile added is such that polyethylene oxide PEO, lithium bistrifluoromethanesulfonimide LiTFSI and graphite phase carbon nitride g-C can be completely dissolved. 3 N 4 A mixture of the three...
Embodiment 3
[0047] This embodiment includes the following steps:
[0048] (1) Add urea to a quartz crucible, and heat it under an air atmosphere at 600 degrees Celsius for 2 hours to obtain graphite-phase carbon nitride g-C with a thinner sheet thickness. 3 N 4 , where the heating rate was 5°C / min.
[0049] (2) the graphite phase carbon nitride g-C prepared in the step (1) 3 N 4 , polyvinylidene fluoride PVDF, and lithium bistrifluoromethanesulfonimide LiTFSI are mixed, and then acetonitrile is added and stirred until the system is uniform to obtain a mixed solution.
[0050] Based on the mass of polyvinylidene fluoride, lithium bistrifluoromethanesulfonimide LiTFSI accounts for 5% of its mass fraction, graphite phase carbon nitride g-C 3 N 4 Accounting for 10% of its mass fraction, the amount of acetonitrile added is enough to completely dissolve polyvinylidene fluoride, lithium bistrifluoromethanesulfonimide LiTFSI and graphite phase carbon nitride g-C 3 N 4 A mixture of the thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com