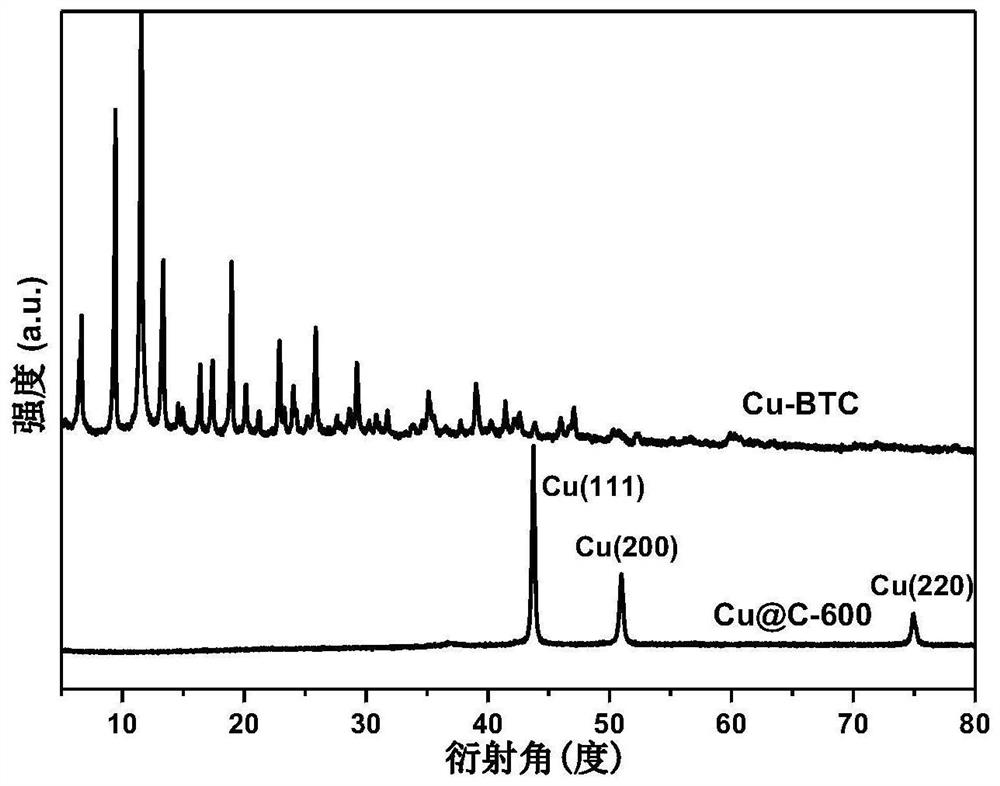
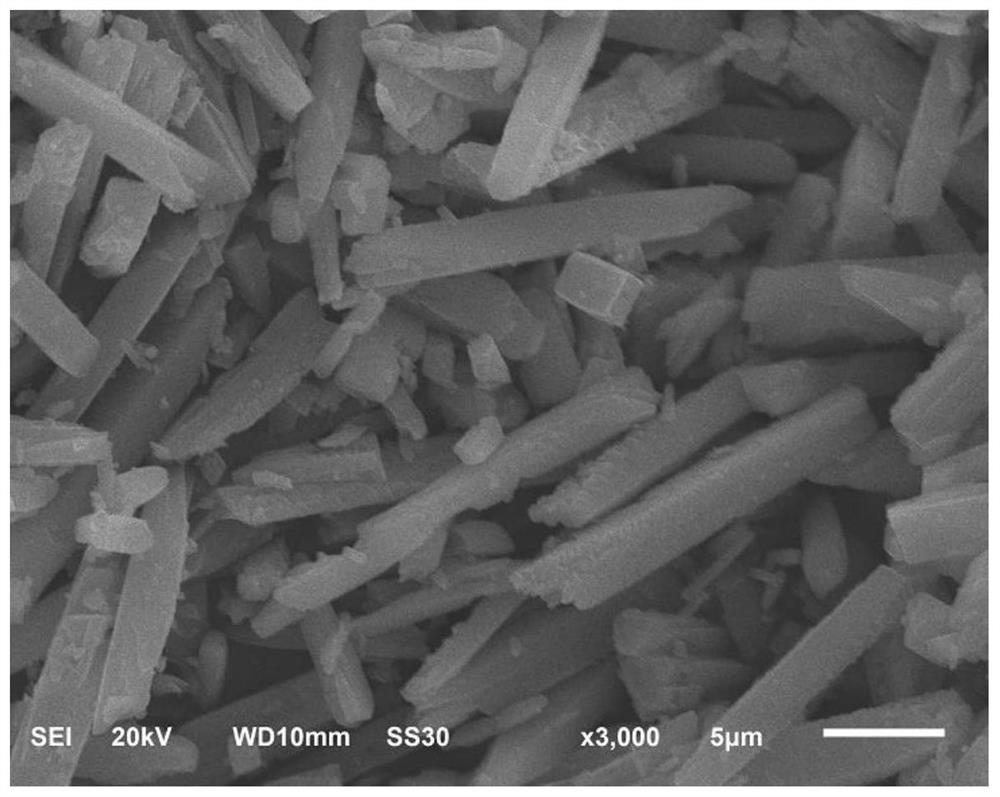
MOF-derived cu@c catalyst and its preparation method and application
A catalyst and reaction technology, applied in the field of tetrahydroquinoline synthesis catalyst, can solve the problems such as the catalyst cannot be recycled for many times, the cost of the catalyst is high, and it is difficult to maintain long-term operation, and the catalyst is cheap, reproducible, and difficult to maintain. High catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035](1) A preparation method of Cu@C catalyst derived from MOF, comprising the steps of:
[0036] 1) Dissolve 0.58mmol trimesic acid (BTC) in 34.8mmol ethanol, 1.12mmol copper nitrate in 89.6mmol water, then mix the above two solutions and stir for 10min, then add 0.0025mmol sodium fluoride and mix Stir at room temperature for 0.5h to carry out pre-synthesis of self-assembly to obtain solution A;
[0037] 2) Add solution A to the polytetrafluoroethylene lining and put it into a stainless steel airtight reaction kettle, then put the sealed reaction kettle into an oven, turn on the heating to 170°C, start the turning mode of the reaction kettle to carry out the rotation crystallization reaction, The turning rate of the reaction kettle is 150rpm / min, and the reaction time is 2h. After the reaction, the reaction kettle is taken out to cool naturally, and the product B is obtained after the kettle is opened;
[0038] 3) The reaction product B was suction-filtered using a Buchner...
Embodiment 2
[0045] (1) A preparation method of Cu@C catalyst derived from MOF, comprising the steps of:
[0046] 1) Dissolve 1.16mmol trimesic acid (BTC) in 58mmol methanol, 3.36mmol copper acetate in 235mmol water, then mix the above two solutions and stir for 30min, then add 0.005mmol potassium fluoride, mix and stir at room temperature 1h for self-assembly pre-synthesis to obtain solution A;
[0047] 2) Add solution A to the polytetrafluoroethylene lining and put it into a stainless steel airtight reaction kettle, then put the sealed reaction kettle into an oven, turn on the heating to 120°C, start the turning mode of the reaction kettle to carry out the rotation crystallization reaction, The turning rate of the reaction kettle is 200rpm / min, and the reaction time is 2h. After the reaction, the reaction kettle is taken out to cool naturally, and the product B is obtained after the kettle is opened;
[0048] 3) The reaction product B was suction-filtered using a Buchner funnel, and the...
Embodiment 3
[0052] (1) A preparation method of Cu@C catalyst derived from MOF, comprising the steps of:
[0053] 1) Dissolve 5.8mmol of 1,2,4-benzenetricarboxylic acid (BTC) in 232mmol of isopropanol and 8.96mmol of copper chloride in 538mmol of water, then mix the above two solutions and stir for 60min, then add 0.025 After mixing mmol ammonium fluoride, stir at room temperature for 2 hours to carry out self-assembly pre-synthesis to obtain solution A;
[0054] 2) Add solution A to the polytetrafluoroethylene lining and put it into a stainless steel airtight reaction kettle, then put the sealed reaction kettle into an oven, turn on the heating to 170°C, start the turning mode of the reaction kettle to carry out the rotation crystallization reaction, The turning rate of the reaction kettle was 56rpm / min, and the reaction time was 2h. After the reaction, the reaction kettle was taken out to cool naturally, and the product B was obtained after the kettle was opened;
[0055] 3) The reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com