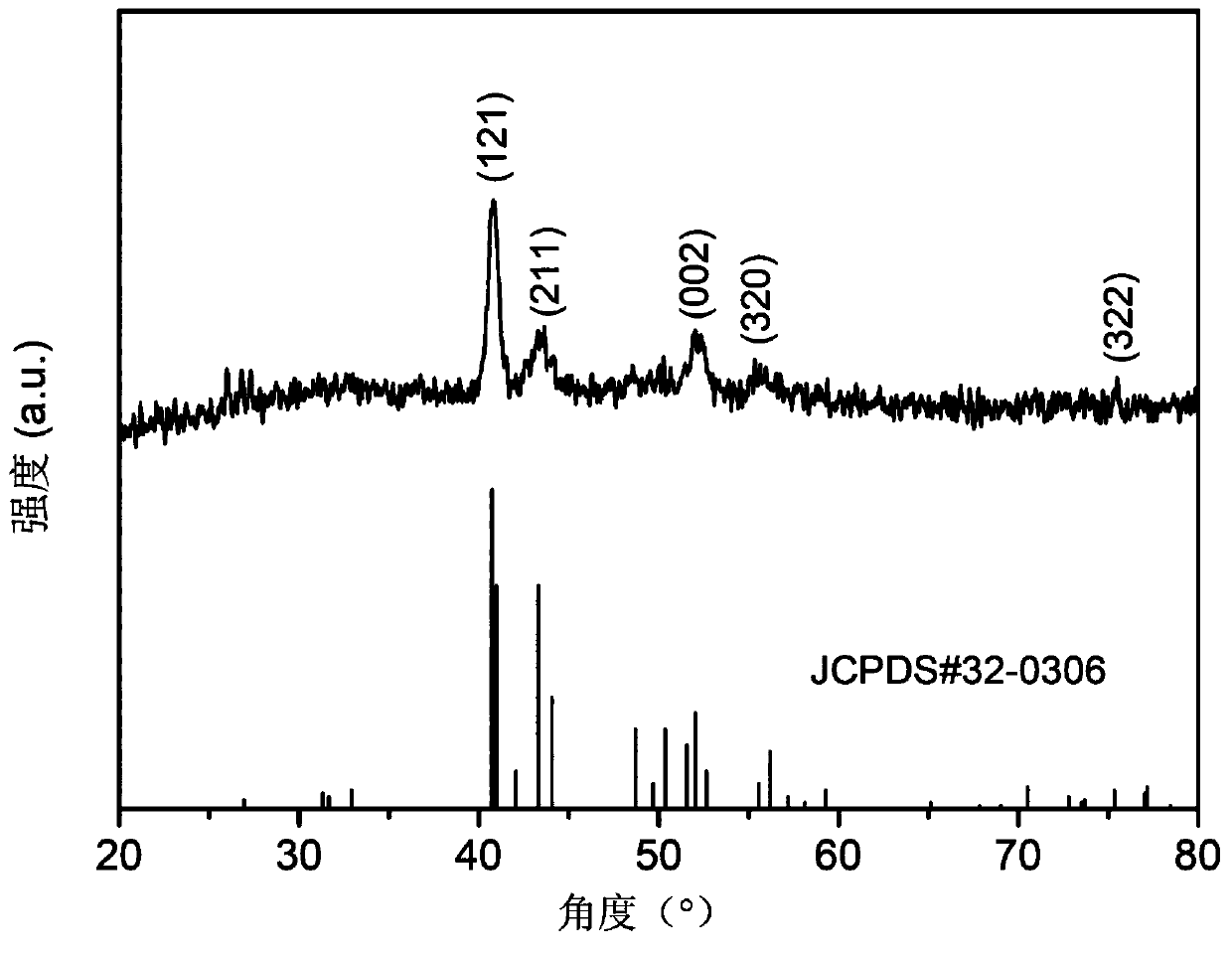
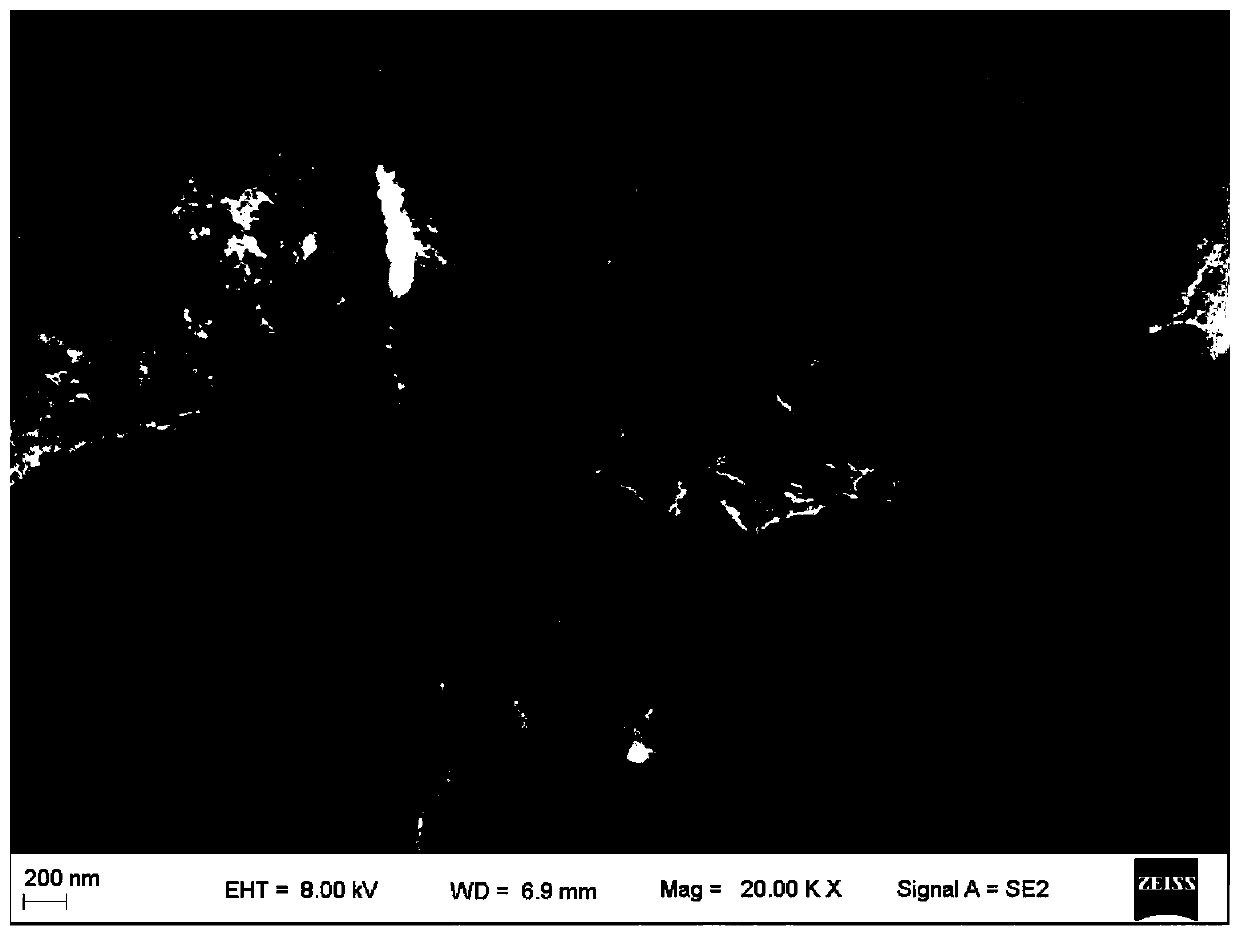
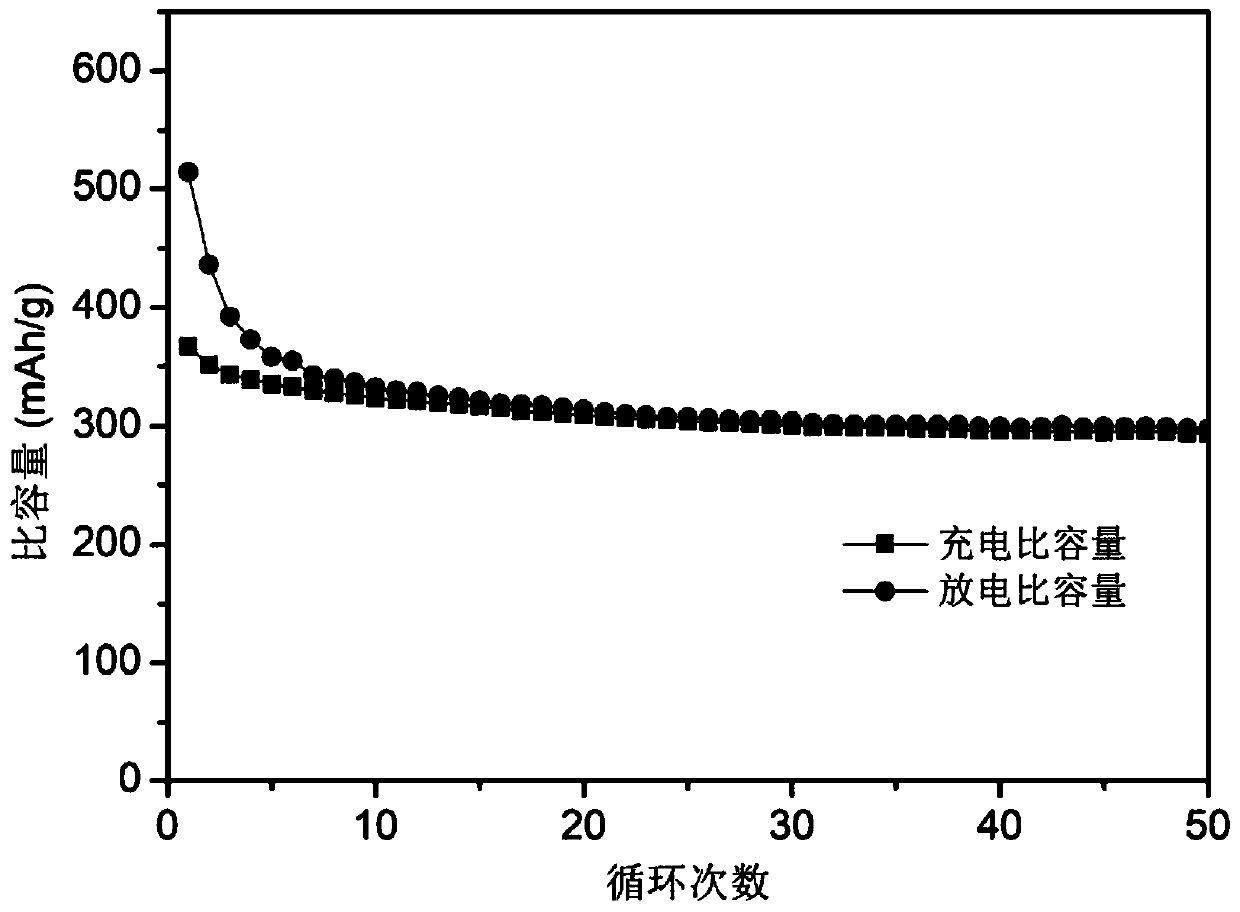
Dicobalt phosphide/carbon composite material, preparation method and use thereof
A carbon composite material, carbon material technology, applied in nanotechnology for materials and surface science, secondary batteries, electrochemical generators, etc. The effect of improving cycle stability and alleviating volume change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] This embodiment prepares Co according to the following method 2 P / C composite material:
[0073] (1) 0.6112g of Co(NO 3 ) 2 ·6H 2 O, 0.3577g of red phosphorus and 0.05g of CTAB surfactant were added to 35mL of deionized water, and magnetically stirred. The obtained suspension was transferred into a 50 mL Teflon-lined stainless steel autoclave, placed in an oven at 210°C, and reacted for 48 hours to obtain Co 2 P precursor. Then wash twice with 3mol / L hydrochloric acid, then wash several times with deionized water and alcohol, and dry in a drying oven.
[0074] Among them, Co(NO 3 ) 2 ·6H 2 The molar ratio of O to red phosphorus is about 1:5.5, and the concentration of surfactant (CTAB) in the suspension system for hydrothermal reaction is 1.43g / L.
[0075] (2) Then take 1g of dry Co 2 The P precursor was added to a 0.048mol / L glucose solution, then transferred to a 50mL Teflon-lined stainless steel autoclave, placed in an oven at 180°C, and reacted for 3 hours...
Embodiment 2
[0085] This embodiment prepares Co according to the following method 2 P / C composite material:
[0086] (1) 0.6112g of Co(NO 3 ) 2 ·6H 2 O, 0.2927g of red phosphorus and 0.1g of CTAB surfactant were added to 35mL of deionized water, and magnetically stirred. The resulting suspension was transferred to a 50 mL Teflon-lined stainless steel autoclave, placed in an oven at 150 ° C, and reacted for 60 h to obtain Co 2 P precursor. Then wash twice with 2mol / L hydrochloric acid, then wash several times with deionized water and alcohol, and dry in a drying oven.
[0087] Among them, Co(NO 3 ) 2 ·6H 2 The molar ratio of O to red phosphorus was 1:4.5, and the concentration of surfactant (CTAB) in the suspension system for hydrothermal reaction was 2.85g / L.
[0088] (2) Then take 1g of dry Co 2 The P precursor was added to a 0.04mol / L glucose solution, then transferred to a 50mL Teflon-lined stainless steel autoclave, placed in an oven at 190°C, and reacted for 4 hours to obtai...
Embodiment 3
[0095] This embodiment prepares Co according to the following method 2 P / C composite material:
[0096] (1) 0.6112g of Co(NO 3 ) 2 ·6H 2 O, 0.3902g of red phosphorus and 0.15g of SDBS surfactant were added to 35mL of deionized water, and magnetically stirred. The obtained suspension was transferred to a 50 mL Teflon-lined stainless steel autoclave, placed in an oven at 220 ° C, and reacted for 30 h to obtain Co 2 P precursor. Then wash twice with 1mol / L hydrochloric acid, then wash several times with deionized water and alcohol, and dry in a drying oven.
[0097] Among them, Co(NO 3 ) 2 ·6H 2 The molar ratio of O to red phosphorus was 1:6, and the concentration of surfactant (SDBS) in the suspension system for hydrothermal reaction was 4.29g / L.
[0098] (2) Then take 1g of dry Co 2 The P precursor was added to a 0.08mol / L glucose solution, then transferred to a 50mL Teflon-lined stainless steel autoclave, placed in an oven at 200°C, and reacted for 2 hours to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com