Total-nutrient formula suitable for enterostomy intestine internal absorption for special medicine
A technology of systemic absorption and complete nutrition, which is applied in the direction of food science, etc., can solve the problems of incomplete decomposition of food, complicated formula process, and no unified standard, so as to enhance immune resistance, shorten hospitalization time, and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
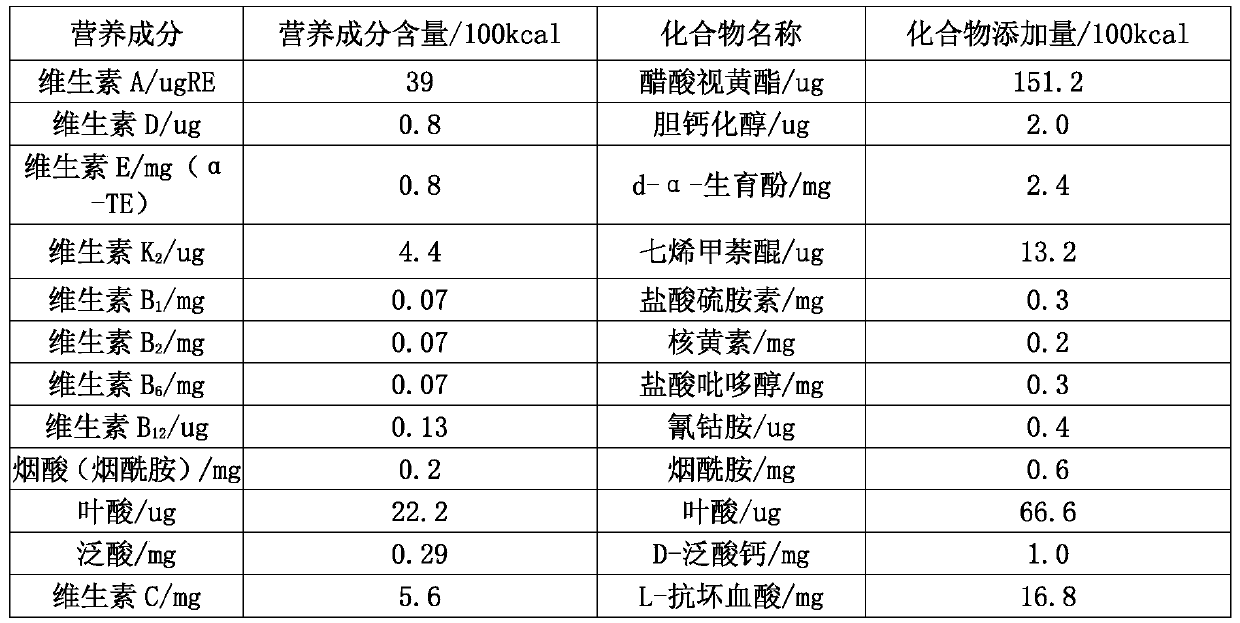
[0022] This formula uses the energy meal alone. It is mainly clinically suitable for daily energy supplementation for patients with gastroenterostomy. The energy meal is brewed with warm water to 250ml, cooled to 35-40°C and injected. The 450kcal energy meal is prepared, and its ingredient ratio is shown in Table 1.
[0023] Table 1 450kcal energy meal preparation plan
[0024] Energy matter
Amount added
Energy matter
Amount added
glucose
15.0g
5.0g
15.0g
10.0g
33.1g
Phytosterols
2.0g
12.0g
soybean oligopeptide
10.0g
resistant dextrin
12.0g
Fish Collagen Peptides
5.0g
675mg
corn peptide
5.0g
225mg
Probiotics (4.5 billion)
45mg
polyethylene glycol
0.2g
0.5g
[0025] Glucose: Sugar source, ...
Embodiment 2
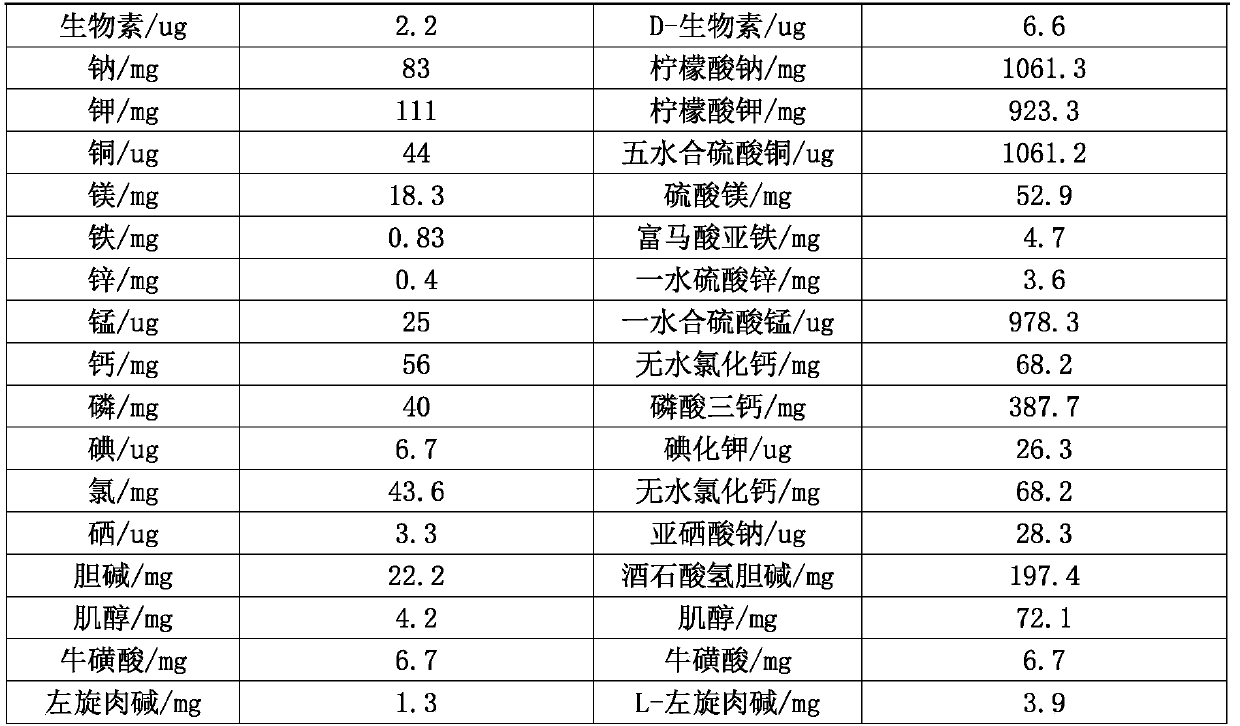
[0039] This formula includes an energy meal (Example 1) and a nutritious meal, which is suitable for daily energy and nutritional supplementation for patients with gastroenterostomy. The intake of the nutritious meal is prepared according to the actual clinical energy intake requirements. In this example, 1 serving of energy meal ( See Table 1) add 4.5 servings of nutritious meal (see Table 2) for preparation, mix the energy meal and nutritious meal, brew in warm water to 250ml, cool to 35-40°C and inject, the preparation ratio of 100kcal nutritious meal is shown in Table 2.
[0040] Table 2 100kcal nutrition package preparation scheme
[0041]
[0042]
Embodiment 3
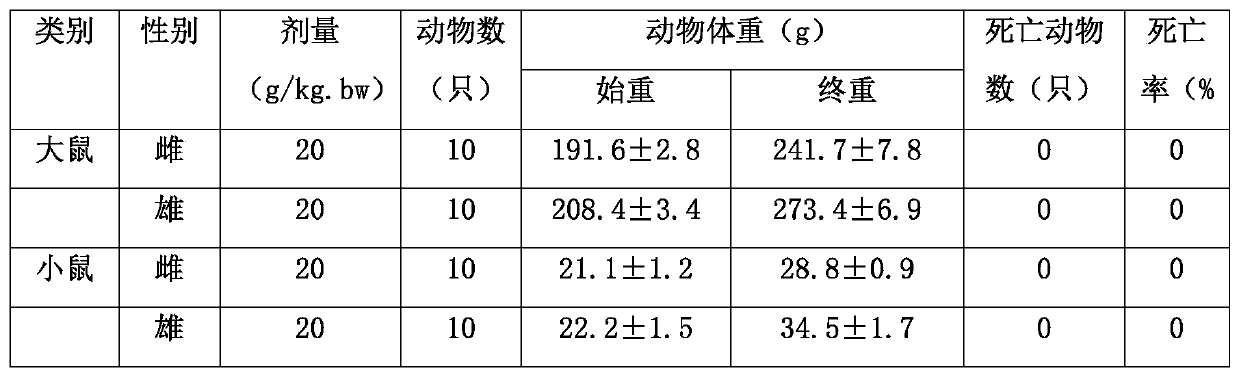
[0044] The maximum tolerated dose method was used in the experiment, and the formula in Example 2 was used as a sample, and a dosage group of 20 g / kg.bw of the sample was set. SD rats and mice, 20 animals each, half male and half female, were given samples by oral gavage, and the sample solution with a concentration of 0.5g / ml was taken, and the samples were given twice at 20ml / kg bw, and the interval between the two times was After 5 hours, give the sample dose equivalent to 20g / kg bw. After giving the sample solution, observe the general condition, body weight change, poisoning symptoms and death of the mice 14 days later. In the acute toxicity test, after the sample liquid was given, no poisoning symptoms and death were seen in rats and mice during the observation period, the body weight all increased, and no adverse reactions were seen, which proves that this product has no acute toxicity and belongs to safe food.
[0045] Table 3 Acute Oral Toxicity Test Results for Rats ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com