Porous boron carbonitride nanosheet layer and porous boron nitride nanosheet layer as well as preparation methods and applications of porous boron carbonitride nanosheet layer and porous boron nitride nanosheet layer as absorbing materials
A nanosheet, boron-carbon-nitrogen technology, applied in the field of nanomaterials, can solve the problems of low capture capacity of h-BN, and achieve the effects of low raw material prices, easy scale-up production, and good process repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
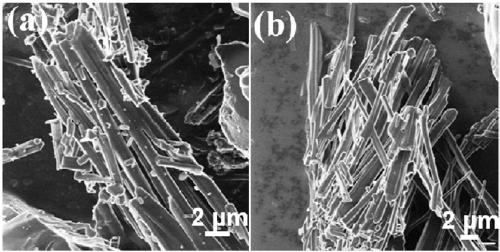
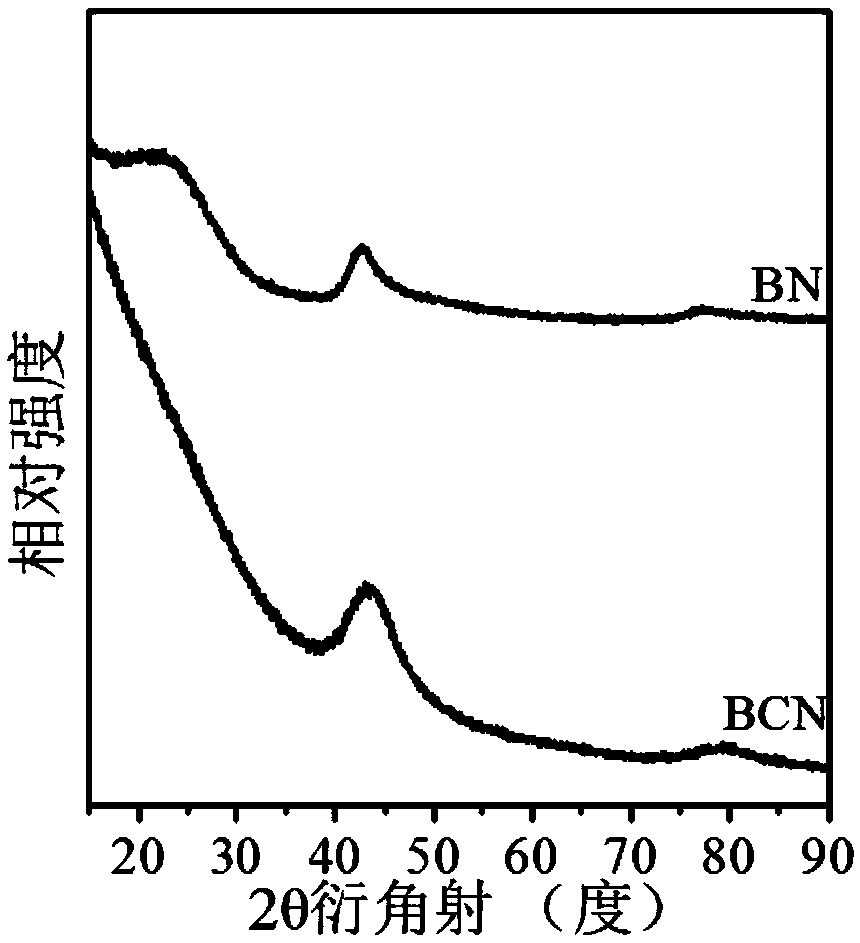
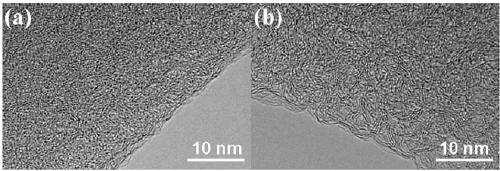
[0071] Preparation of porous boron carbon nitrogen (BCN) nanosheets: Weigh 3.0914g of boric acid into a round-bottomed flask, add 300mL of distilled water, place the round-bottomed flask in a 60°C oil bath and heat to a constant temperature. After the boric acid is completely dissolved, weigh Measure 3.1541g of melamine into the round bottom flask, wherein the molar ratio of boric acid: melamine is 2:1, add a rubber stopper to the mouth of the round bottom flask, stir at 60°C for 3 hours, then raise the temperature of the oil bath to 90°C, stir for 8h, Then remove the bottle stopper and dry it with stirring at 90°C. The dried solid was taken out of a mortar and ground to a fine powder. Add the ground solid powder into a quartz boat, put it into a tube furnace, raise the temperature to 800°C at 2°C / min under an argon atmosphere, keep it for 6 hours, and drop it to room temperature under an argon atmosphere. The obtained sample is BCN material.
[0072] Raise the temperature o...
Embodiment 2
[0079] Preparation of porous boron carbon nitrogen (BCN) nanosheets: Weigh 3.0899g of boric acid into a round-bottomed flask, add 300mL of distilled water, place the round-bottomed flask in a 60°C oil bath and heat to a constant temperature. After the boric acid is completely dissolved, weigh Measure 6.3047g of melamine into the round bottom flask, in which the molar ratio of boric acid: melamine is 1:1, put a rubber stopper on the mouth of the round bottom flask, stir at 60°C for 2 hours, heat the oil bath to 100°C, and stir for 7h , and then remove the bottle stopper, and stir and dry at 100°C. The dried solid was taken out of a mortar and ground to a fine powder. Put the ground solid powder into a quartz boat, put it into a tube furnace, raise the temperature to 800°C at 10°C / min under an argon atmosphere, keep it for 4 hours, and then drop it to room temperature under an argon atmosphere. The obtained sample is Porous boron carbon nitrogen (BCN) nanosheets.
[0080] The ...
Embodiment 3
[0084] Preparation of porous boron carbon nitrogen (BCN) nanosheets: Weigh 3.0673g of boric acid into a round-bottomed flask, add 300mL of distilled water, place the round-bottomed flask in a 60°C oil bath and heat to a constant temperature. After the boric acid is completely dissolved, weigh Measure 6.3221g of melamine into the round bottom flask, wherein the molar ratio of boric acid: melamine is 1:1, put a rubber stopper on the mouth of the round bottom flask, stir at 60°C for 3 hours, heat the oil bath to 90°C, stir After 8 hours, remove the bottle stopper and dry it with stirring at 100°C. The dried solid was taken out of a mortar and ground to a fine powder. Add the ground solid powder into a quartz boat, put it into a tube furnace, raise the temperature to 700°C at 5°C / min in an argon atmosphere, keep it for 6 hours, and drop it to room temperature under argon atmosphere to obtain a sample as follows: Porous boron carbon nitrogen (BCN) nanosheets.
[0085] The porous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mesopore | aaaaa | aaaaa |
| micropore | aaaaa | aaaaa |
| adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com