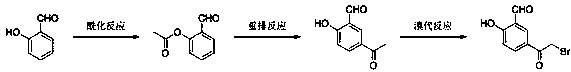
Synthesis method of 5-(2-bromoacetyl)-2-hydroxybenzaldehyde
A technology of hydroxybenzaldehyde and bromoacetyl group is applied in the synthesis field of 5--2-hydroxybenzaldehyde, can solve the problems of high price of bromoacetyl bromide, poor selectivity, restricting industrial production and the like, and achieves reduction of purification difficulty and improvement of yield. efficiency and purity, and the availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 2-(acetoxy)-benzaldehyde:
[0020] Add glacial acetic acid (50 mL) into a 500 mL three-necked flask, add salicylaldehyde (24.4 g, 0.2 mol) and acetic anhydride (22.5 g, 0.22 mol) dropwise into glacial acetic acid under stirring, Under reflux for 2 h, the TLC control reaction was completed. The reaction solution was added to 100 mL of ice water, cooled to 5°C, stirred for 1-2 h, and separated by filtration after solid precipitation. Finally, it was dissolved in 125 mL of ethyl acetate, dried over anhydrous magnesium sulfate, and the ethyl acetate was concentrated in vacuo to obtain 32.6 g of solid 2-(acetoxy)-benzaldehyde with a yield of 99.4%.
[0021] Preparation of 5-acetyl-2-hydroxy-benzaldehyde:
[0022] Add dichloromethane (250 mL) into a 500 mL three-necked flask, add anhydrous aluminum trichloride (36 g, 0.27 mol) under stirring, raise the temperature to 25 °C, and then add 2-(acetoxy)-benzaldehyde ( 29.5 g, 0.18 mol), kept at 25°C, reacted for ...
Embodiment 2
[0026] Preparation of 2-(acetoxy)-benzaldehyde:
[0027] Add glacial acetic acid (50 mL) into a 500 mL three-necked flask, and add salicylaldehyde (24.4 g, 0.2 mol) and acetyl chloride (18.8 g, 0.24 mol) dropwise into glacial acetic acid under stirring. Under reflux for 2 h, the TLC control reaction was completed. The reaction solution was added to 100 mL of ice water, cooled to 5°C, stirred for 1-2 h, and separated by filtration after solid precipitation. Finally, it was dissolved in 125 mL of ethyl acetate, dried over anhydrous magnesium sulfate, and the ethyl acetate was concentrated in vacuo to obtain 32.2 g of solid 2-(acetoxy)-benzaldehyde with a yield of 98.2%.
[0028] Preparation of 5-acetyl-2-hydroxy-benzaldehyde:
[0029] Add dichloromethane (250 mL) into a 500 mL three-necked flask, add zinc chloride (34.1 g, 0.25 mol) while stirring, raise the temperature to 30 °C, and then add 2-(acetoxy)-benzaldehyde (29.5 g, 0.18 mol), kept at 30°C, reacted for 10 h, and the...
Embodiment 3
[0033] Preparation of 2-(acetoxy)-benzaldehyde:
[0034] Add glacial acetic acid (50 mL) into a 500 mL three-necked flask, and add dropwise salicylaldehyde (24.4 g, 0.2 mol) and acetic anhydride (24.5 g, 0.24 mol) into glacial acetic acid under stirring. Under reflux for 2 h, the TLC control reaction was completed. The reaction solution was added to 100 mL of ice water, cooled to 5°C, stirred for 1-2 h, and separated by filtration after solid precipitation. Finally, it was dissolved in 125 mL of ethyl acetate, dried with anhydrous magnesium sulfate, and the ethyl acetate was concentrated in vacuo to obtain 32.3 g of solid 2-(acetoxy)-benzaldehyde with a yield of 98.5%.
[0035] Preparation of 5-acetyl-2-hydroxy-benzaldehyde:
[0036] Add dichloromethane (250 mL) into a 500 mL three-necked flask, add anhydrous ferric chloride (40.6 g, 0.25 mol) while stirring, raise the temperature to 25 °C, and then add 2-(acetoxy)-benzaldehyde (29.5 g, 0.18 mol), kept at 25°C, reacted for 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com