Hydroxyproline peptoid derivative and preparation method and application thereof
A peptide derivative, hydroxyproline technology, used in drug combination, organic chemistry, anti-tumor drugs, etc., can solve problems such as adverse reactions and drug resistance, and achieve improved affinity, improved inhibitory activity, and good spatial matching. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
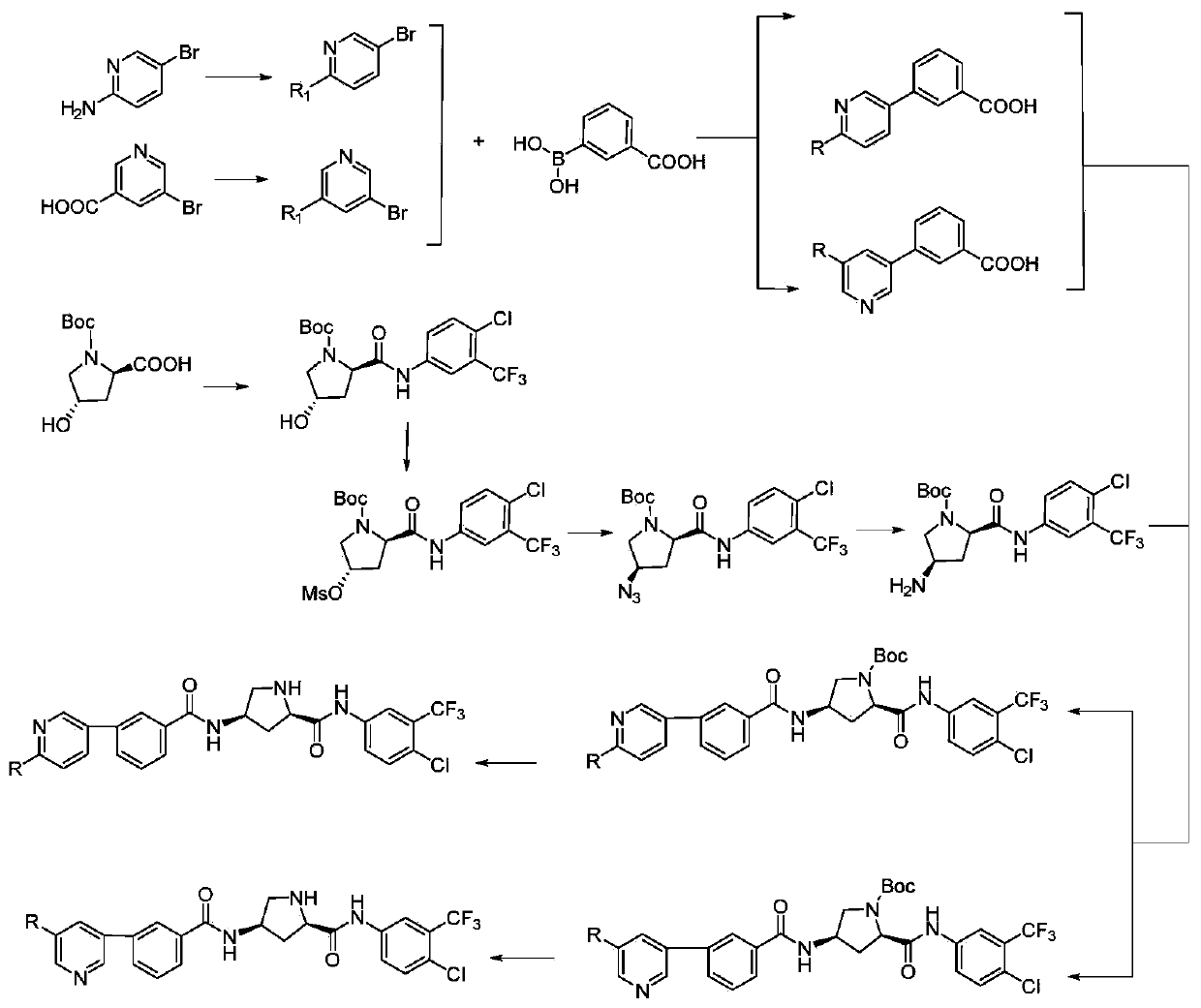
[0035] see figure 1 , as the preparation method of the hydroxyproline peptide derivatives of the above structure, comprising the following steps:
[0036] 1) 5-bromo-2-aminopyridine is acylated with an acid chloride compound to prepare acylated 5-bromo-2-aminopyridine;
[0037] 2)N 2 Under protection, 5-bromo-nicotinic acid reacts with thionyl chloride and amine compounds to prepare ammoniated 5-bromo-nicotinic acid;
[0038]3) Under the catalysis of tetrakistriphenylphosphine palladium, acylated 5-bromo-2-aminopyridine or ammoniated 5-bromonicotinic acid undergoes Suzuki coupling reaction with m-carboxyphenylboronic acid to obtain biphenyl compounds;
[0039] 4) Condensation of Boc-protected hydroxyproline with 3-trifluoromethyl-4-chloroaniline to generate tert-butyl-(2R,4S)-2-((4-chloro-3-(trifluoromethyl)benzene Base) carbamoyl)-4-hydroxypyrrolidinyl-1-carboxylate;
[0040] 5) tert-butyl-(2R,4S)-2-((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)-4-hydroxypyrrolidinyl-1-c...
Embodiment 1
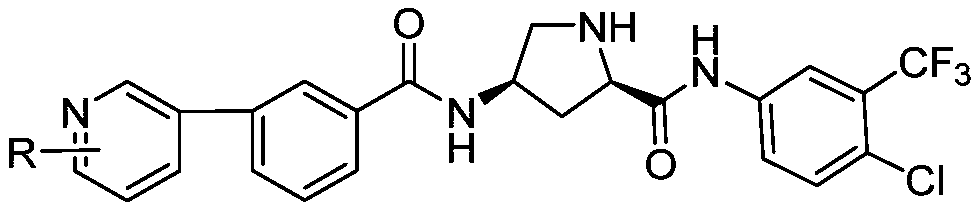
[0055] A hydroxyproline peptide derivative, characterized in that R is , the preparation method is as follows:
[0056] 1) Synthesis of N-(5-bromopyridin-2-yl)acetamide: 5-bromo-2-aminopyridine (5.19 g, 30 mmol) was dissolved in 100 ml of anhydrous dichloromethane, and 20 ml of triethylamine was added. Under the condition of ice bath, acetyl chloride (2.54ml) was slowly added dropwise to the above solution. After the dropwise addition was completed, the ice bath was removed, and the mixture was raised to room temperature to react overnight. After the reaction was finished, dichloromethane was added for dilution, washed with water (30ml×3), saturated NaHCO 3 Solution washing (30ml×3), saturated NaCl washing (30ml), organic phase anhydrous NaCl 2 SO 4 dry. Column chromatography separated 5.65 g of white solid with a yield of 88%. Mp 78-81℃; EI-MS(m / z):214[M] + .
[0057] 2) Synthesis of 4-(6-(acetylamino)pyridin-3-yl)benzoic acid: N-(5-bromopyridin-2-yl)acetamide (4.30g,...
Embodiment 2
[0064] A hydroxyproline peptide derivative, characterized in that R is , the preparation method is as follows:
[0065] 1) Synthesis of 5-bromo-N-cyclopropyl nicotinamide: in N 2 Under protection, add thionyl chloride (36ml, 494mmol) dropwise to 5-bromonicotinic acid (5.00g, 24.7mmol). After the dropwise addition, heat to reflux for 2-3h until the solution is clear, and spin off the chloride under reduced pressure. Sulfoxide, a light yellow solid was obtained. The solid was dissolved in 30ml of anhydrous dichloromethane, and the reactive intermediate solution was slowly added dropwise to a solution of cyclopropylamine (3.77ml) in dichloromethane (30ml) in an ice bath. After the dropwise addition, it was raised to room temperature and reacted overnight. After the reaction, add 2mol / L K to the reaction system 2 CO 3 Solution 20ml. Separate the liquid to take the dichloromethane phase, extract the aqueous phase with dichloromethane (15ml × 3), combine the organic phases, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com