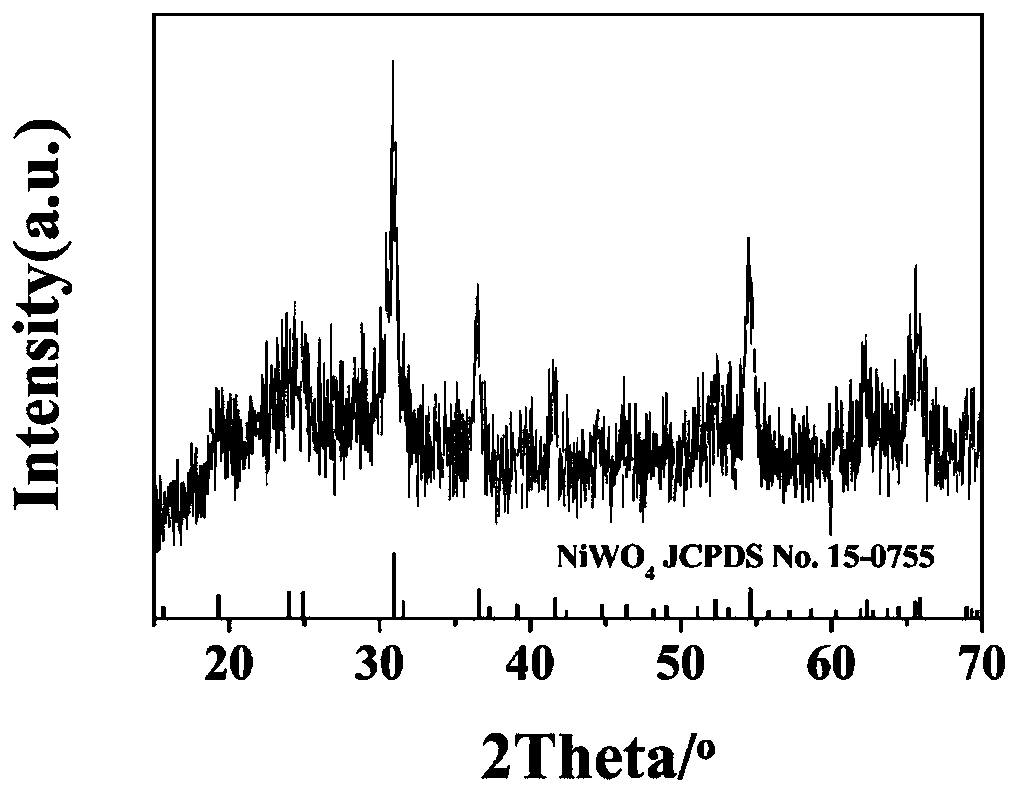
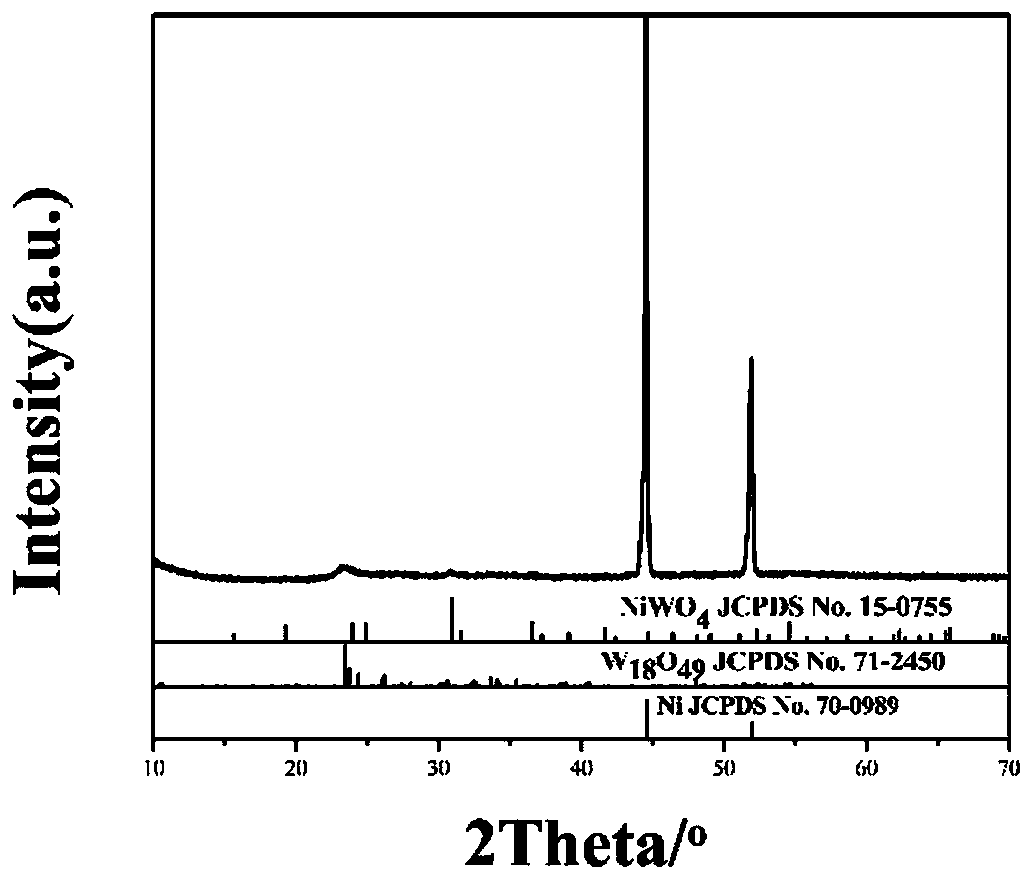
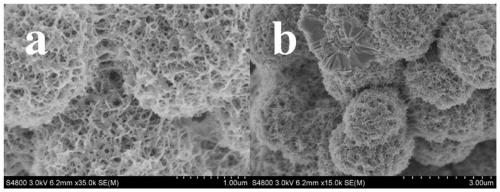
Preparation method of W18O49/NiWO4/NF self-supporting electrocatalytic material
An electrocatalytic material and self-supporting technology, applied in chemical instruments and methods, physical/chemical process catalysts, electrodes, etc., can solve problems such as instability, low specific surface area, poor conductivity, etc., and achieve process controllability, reaction The effect of low temperature and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Take Na at a molar ratio of 1:1 2 WO 4 2H 2 O and Ni(CH 3 COO)2 4H 2 O, the Na 2 WO 4 2H 2 O and Ni(CH 3 COO) 2 4H 2 O was added to 40mL deionized water and stirred evenly to obtain Na 2 WO 4 2H 2 O concentration is 0.05mol / L, Ni(CH 3 COO) 2 4H 2 O concentration is the mixed solution A of 0.05mol / L;
[0034] 2) Put the mixed solution A into a polytetrafluoroethylene-lined autoclave with a volume filling ratio of 40%, seal the autoclave and put it into a homogeneous hydrothermal reactor for 12 hours at 160°C;
[0035] 3) Cool to room temperature after the reaction, centrifuge and wash the reactant three times with absolute ethanol and deionized water respectively, and dry the reactant after centrifugal washing in a vacuum oven or freeze drying oven at 50°C for 5 hours to obtain powder B;
[0036] 4) Grind powder B in a mortar and put it into a muffle furnace to heat up from room temperature to 450°C at a heating rate of 2°C / min for calcination to obtai...
Embodiment 2
[0045] 1) Take Na2WO4·2H at a molar ratio of 1:1 2 O and Ni(CH 3 COO) 2 4H 2 O, the Na 2 WO4·2H 2 O and Ni(CH 3 COO) 2 4H 2 O was added to 30mL deionized water and stirred evenly to obtain Na 2 WO 4 2H 2 O concentration is 0.03mol / L, Ni(CH 3 COO) 2 4H 2 O concentration is the mixed solution A of 0.03mol / L;
[0046] 2) Put the mixed solution A into a polytetrafluoroethylene-lined autoclave with a volume filling ratio of 30%, seal the autoclave and put it into a homogeneous hydrothermal reactor for 12 hours at 180°C;
[0047] 3) Cool to room temperature after the reaction, centrifuge and wash the reactant three times with absolute ethanol and deionized water respectively, and dry the reactant after centrifugal washing in a vacuum oven or freeze drying oven at 50°C for 5 hours to obtain powder B;
[0048] 4) Grind powder B in a mortar and put it into a muffle furnace to heat up from room temperature to 470°C at a heating rate of 2°C / min for calcination to obtain NiW...
Embodiment 3
[0057] 1) Take Na at a molar ratio of 1:1 2 WO 4 2H 2 O and Ni(CH 3 COO) 2 4H 2 O, the Na 2 WO 4 2H 2 O and Ni(CH 3 COO) 2 4H 2 O was added to 35mL deionized water and stirred evenly to obtain Na 2 WO 4 2H 2 O concentration is 0.08mol / L, Ni(CH 3 COO) 2 4H 2 O concentration is the mixed solution A of 0.08mol / L;
[0058] 2) Put the mixed solution A into a polytetrafluoroethylene-lined autoclave with a volume filling ratio of 35%, seal the autoclave and put it into a homogeneous hydrothermal reactor for 28 hours at 150°C;
[0059] 3) Cool to room temperature after the reaction, centrifuge and wash the reactant three times with absolute ethanol and deionized water respectively, and dry the reactant after centrifugal washing in a vacuum oven or freeze drying oven at 50°C for 6 hours to obtain powder B;
[0060] 4) Grind powder B in a mortar and put it into a muffle furnace to heat up from room temperature to 450°C at a heating rate of 2°C / min for calcination to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com