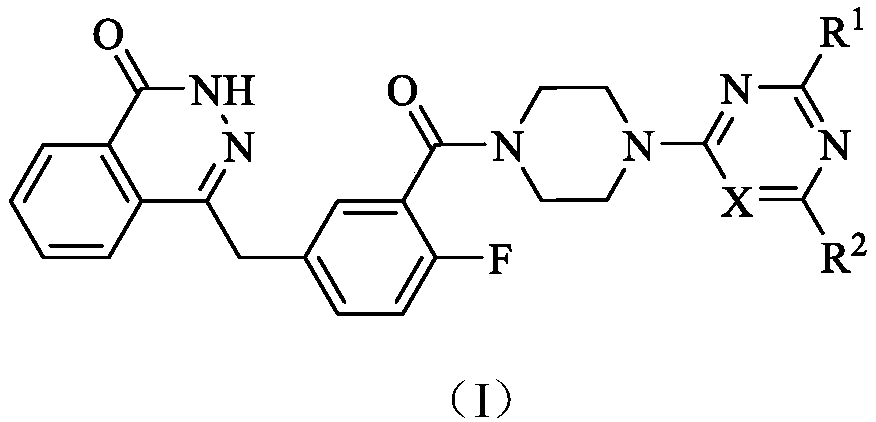
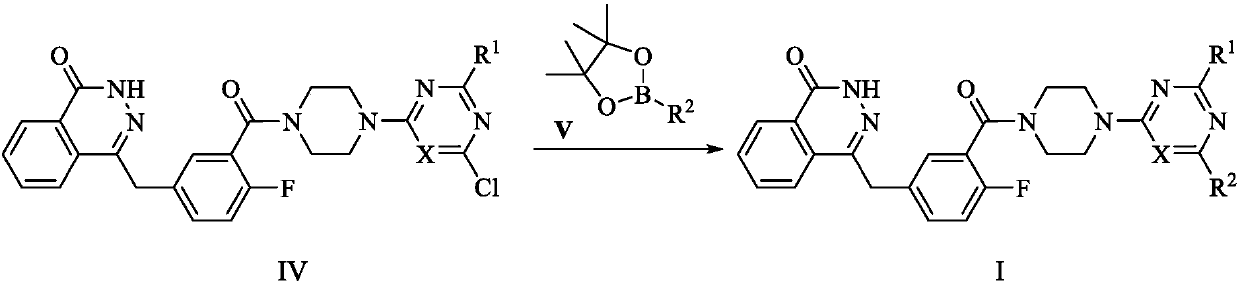
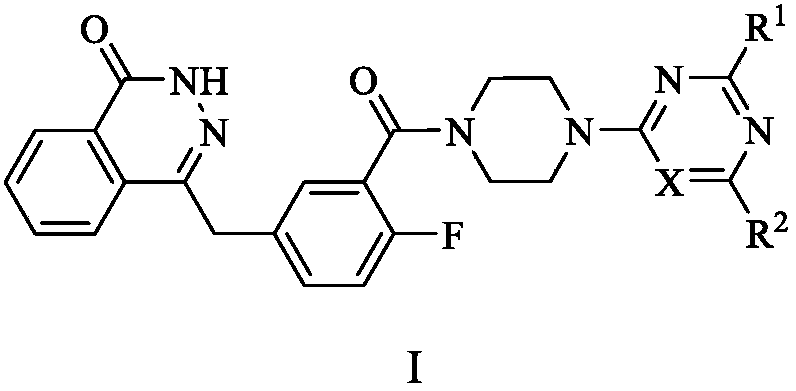
PARP (poly-ADP-ribose polymerase)-1 and PI3K (phosphatidylinositol-3-kinase) dual target inhibitors containing phthalazine-1(2H)-ketone structure
A PARP-1, CH3 technology, applied in the fields of medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., can solve the problem of no dual-target inhibitor reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 4-(3-(4-(4-(2-aminopyrimidin-5-yl)-6-morpholinyl-1,3,5-triazin-2-yl)piperazine-1-formyl)- Synthesis of 4-fluorobenzyl)phthalazin-1(2H)-one (I-1)
[0066] (2,6-dichloro-4-morpholinyl)-1,3,5-triazine (2)
[0067] Dissolve tripolychlorazine (1) (10.00g, 54.23mmol) in 100mL of dichloromethane, add DIEA (9.92mL, 56.94mmol), cool to -78°C, dissolve morpholine (4.73mL, 54.23mmol) The solution in 10mL of dichloromethane was added dropwise to the reaction solution. After the addition was complete, a large amount of white solids were precipitated. TLC (petroleum ether: ethyl acetate = 6:1) detected that the reaction of raw material 1 was completed, and the reaction was stopped. Suction filtration, the filter cake was water After washing and drying, 7.18 g of white solid was obtained, with a yield of 56.4%. 1 H NMR (300MHz, DMSO-d 6 )δ(ppm):3.80-3.76(4H,CH 2 O),3.69(4H,CH 2 ).
[0068] tert-butyl 4-(4-chloro-6-morpholinyl-1,3,5-triazin-2-yl)piperazine-1-carboxylate (3)
[0...
Embodiment 2
[0077] 4-(3-(4-(4-(6-aminopyridin-3-yl)-6-morpholinyl-1,3,5-triazin-2-yl)piperazine-1-formyl)- Synthesis of 4-fluorobenzyl)phthalazin-1(2H)-one (I-2)
[0078] With compound IV-1 (300mg, 0.53mmol) and 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine-2- Amine (V-2) (128mg, 0.58mmol) was used as a raw material, and the operation was the same as that of I-1. It was separated by column chromatography (eluent: dichloromethane:methanol=80:1~30:1 gradient elution), and obtained shallow Yellow solid 160 mg, yield 48.5%. m.p.180-182℃. 1 HNMR (300MHz, DMSO-d 6 )δ (ppm): 12.61 (1H, s, CONH), 8.89 (1H, s, ArH), 8.26-8.19 (2H, m, ArH), 7.97-7.79 (3H, m, ArH), 7.46-7.37 ( 2H, m, ArH), 7.24 (1H, t, J=8.7Hz, ArH), 6.57 (2H, s, ArNH 2 ), 6.45 (1H, d, J=8.4Hz, ArH), 4.33 (2H, s, ArCH 2 ),3.93-3.64(14H,m,2CH 2 O,5CH 2 N),3.27-3.18(2H,m,CH 2 N). 13 C-NMR (75MHz, DMSO-d 6 )δ(ppm):168.62,164.31,163.98,161.91,159.34,154.76,149.69,144.82,136.66,134.76,133.44,131.49,129.05,127.88,126....
Embodiment 3
[0080] 4-(3-(4-(4-(6-Amino-4-(trifluoromethyl)pyridin-3-yl)-6-morpholinyl-1,3,5-triazin-2-yl) Synthesis of piperazine-1-formyl)-4-fluorobenzyl)phthalazin-1(2H)-one (I-3)
[0081] With compound IV-1 (300mg, 0.53mmol) and 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-4-( Trifluoromethyl)pyridin-2-amine (V-3) (168mg, 0.58mmol) is raw material, operation is the same as I-1, column chromatography separates (eluent: dichloromethane:methanol=80:1~30 : 1 gradient elution), to obtain off-white solid 185mg, yield 50.6%. m.p.159-161℃. 1 H NMR (300MHz, DMSO-d 6 )δ (ppm): 12.61 (1H, s, CONH), 8.61 (1H, s, ArH), 8.27 (1H, d, J = 7.4Hz, ArH), 7.97 (1H, d, J = 7.4Hz, ArH ),7.91-7.80(2H,m,ArH),7.47-7.43(1H,m,ArH),7.39(1H,dd,J=6.4,1.9Hz,ArH),7.25(1H,t,J=9.0Hz ,ArH),6.97(2H,s,NH 2 ),6.82(1H,s,ArH),4.34(2H,s,ArCH 2 ),3.85-3.63(14H,m,2CH 2 O,5CH 2 N),3.24-3.21(2H,m,CH 2 N). 13 C-NMR (75MHz, DMSO-d 6 )δ(ppm):169.46,164.03,161.18,159.34,158.00,154.75,152.56,144.81,135.87,134.81,133.40,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com