Chlorine dioxide solid releasing agent and preparation method thereof
A technology of chlorine dioxide and release agent, which is applied in botany equipment and methods, biocides, disinfectants, etc., can solve the problems of slow release, long activation time, and large loss rate, so as to reduce the formation of surface moisture and protect the environment Safe, no secondary pollution, strong oxidation and sterilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
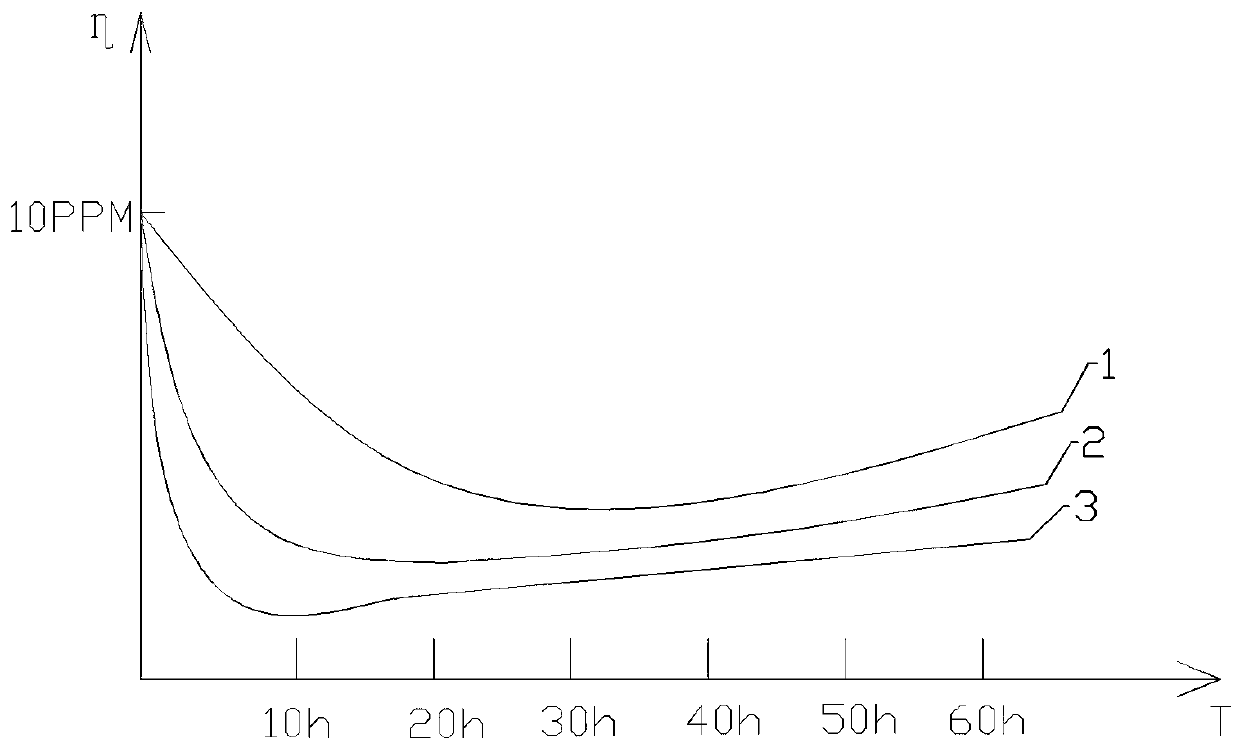
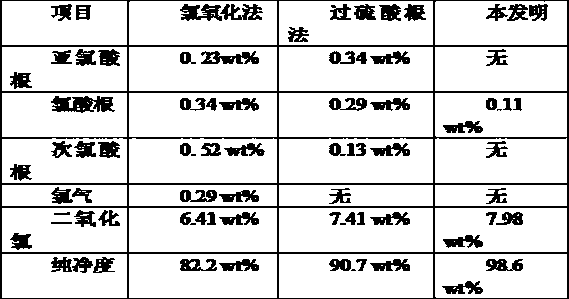
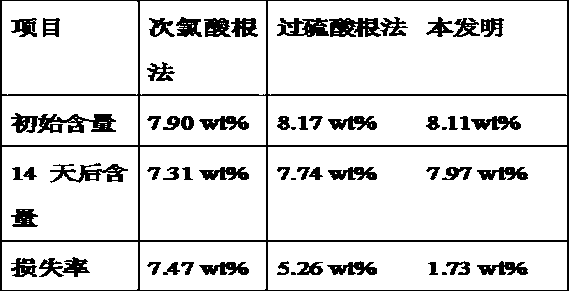
Image
Examples
Embodiment 1
[0041] A chlorine dioxide solid release agent, made of the following raw materials in parts by weight, sodium chlorite 10Kg, anhydrous sodium bisulfate 50Kg, potassium bromide 2Kg, potassium bromate 1Kg, anhydrous magnesium sulfate 5Kg, anhydrous sodium sulfate 25Kg, sodium carbonate 7Kg.
[0042] The preparation method is as follows:
[0043] Step 1, taking by weighing 10Kg of sodium chlorite, 5Kg of anhydrous magnesium sulfate, 25Kg of anhydrous sodium sulfate, and 7Kg of sodium carbonate, and mixing the four to obtain a mixed powder;
[0044] Step 2, taking by weighing 50Kg of anhydrous sodium bisulfate, 2Kg of potassium bromide, and 1Kg of potassium bromate, and mixing the three to obtain a bromine-containing inorganic salt activator;
[0045] Step 3, mixing the mixed powder with a bromine-containing inorganic salt activator to obtain a powdery chlorine dioxide solid release agent;
[0046] Step 4, compressing the powdered chlorine dioxide solid release agent into tablet...
Embodiment 2
[0048] A chlorine dioxide solid release agent, made of the following raw materials in parts by weight, sodium chlorite 8Kg, sodium bisulfate 40Kg, sodium bicarbonate 20 Kg, boric acid 5Kg, potassium bromide 2.5Kg, potassium bromate 0.5Kg, Anhydrous magnesium sulfate 5Kg, anhydrous sodium sulfate 10Kg, anhydrous calcium chloride 9Kg.
[0049] The preparation method is as follows:
[0050] Step 1, taking by weighing 8Kg of sodium chlorite, 5Kg of anhydrous magnesium sulfate, 10Kg of anhydrous sodium sulfate, and 9Kg of anhydrous calcium chloride, and mixing the four to obtain a mixed powder;
[0051] Step 2, take by weighing 40 Kg of sodium bisulfate, 20 Kg of sodium bicarbonate, 5Kg of boric acid, 2.5Kg of potassium bromide, 0.5Kg of potassium bromate, and mix the five to obtain a bromine-containing inorganic salt activator;
[0052] Step 3, mixing the mixed powder with a bromine-containing inorganic salt activator to obtain a powdery chlorine dioxide solid release agent;
[...
Embodiment 3
[0055] A chlorine dioxide solid release agent, made of the following raw materials in parts by weight, sodium chlorite 13Kg, sodium bisulfate 50Kg, sodium bicarbonate 10 Kg, potassium bromide 3.5Kg, potassium bromate 1.5Kg, anhydrous magnesium sulfate 5Kg, anhydrous sodium metasilicate 5Kg, anhydrous magnesium chloride 7Kg.
[0056] The preparation method is as follows:
[0057] Step 1, weighing 13Kg of sodium chlorite, 5Kg of anhydrous magnesium sulfate, 5Kg of anhydrous sodium metasilicate, and 7Kg of anhydrous magnesium chloride, and mixing the four to obtain a mixed powder;
[0058] Step 2, taking by weighing sodium bisulfate 50Kg, sodium bicarbonate 10 Kg, potassium bromide 3.5Kg, potassium bromate 1.5Kg, four are mixed, obtain bromine-containing inorganic salt activator;
[0059] Step 3, mixing the mixed powder with a bromine-containing inorganic salt activator to obtain a powdery chlorine dioxide solid release agent;
[0060] Step 4, compressing the powdered chlorine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com