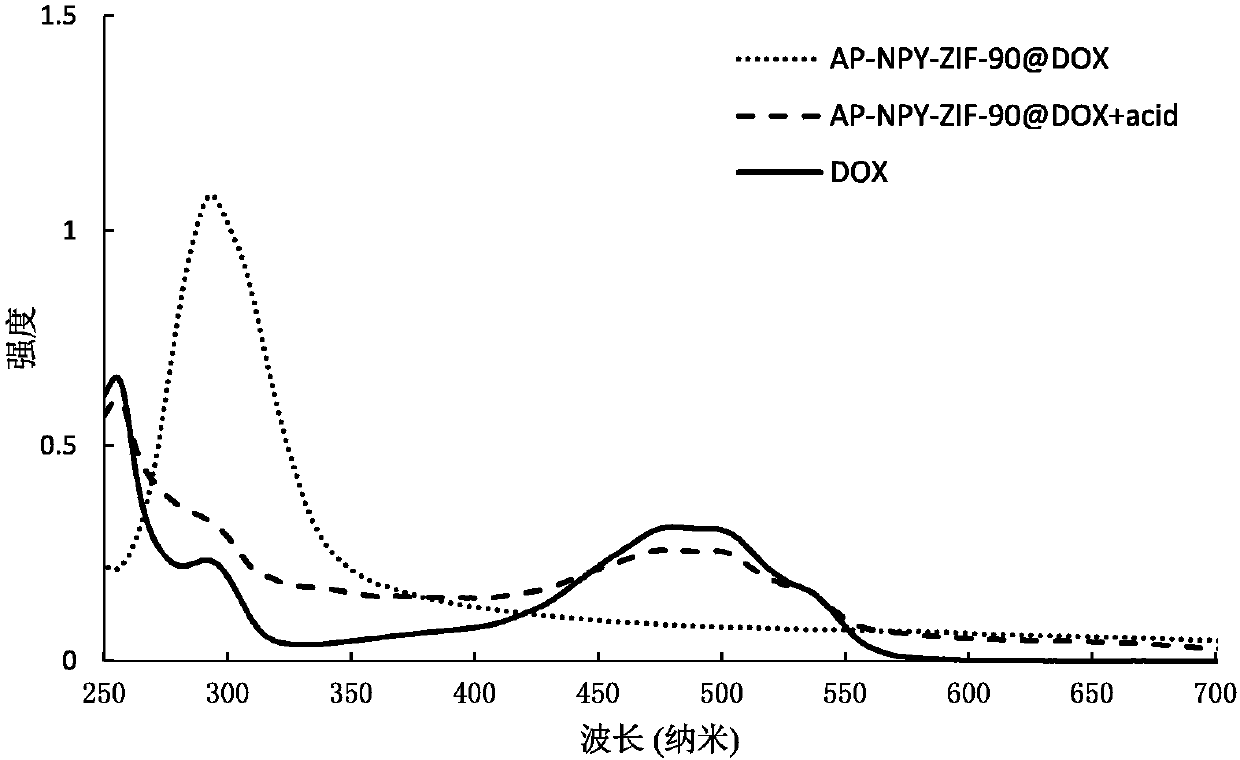
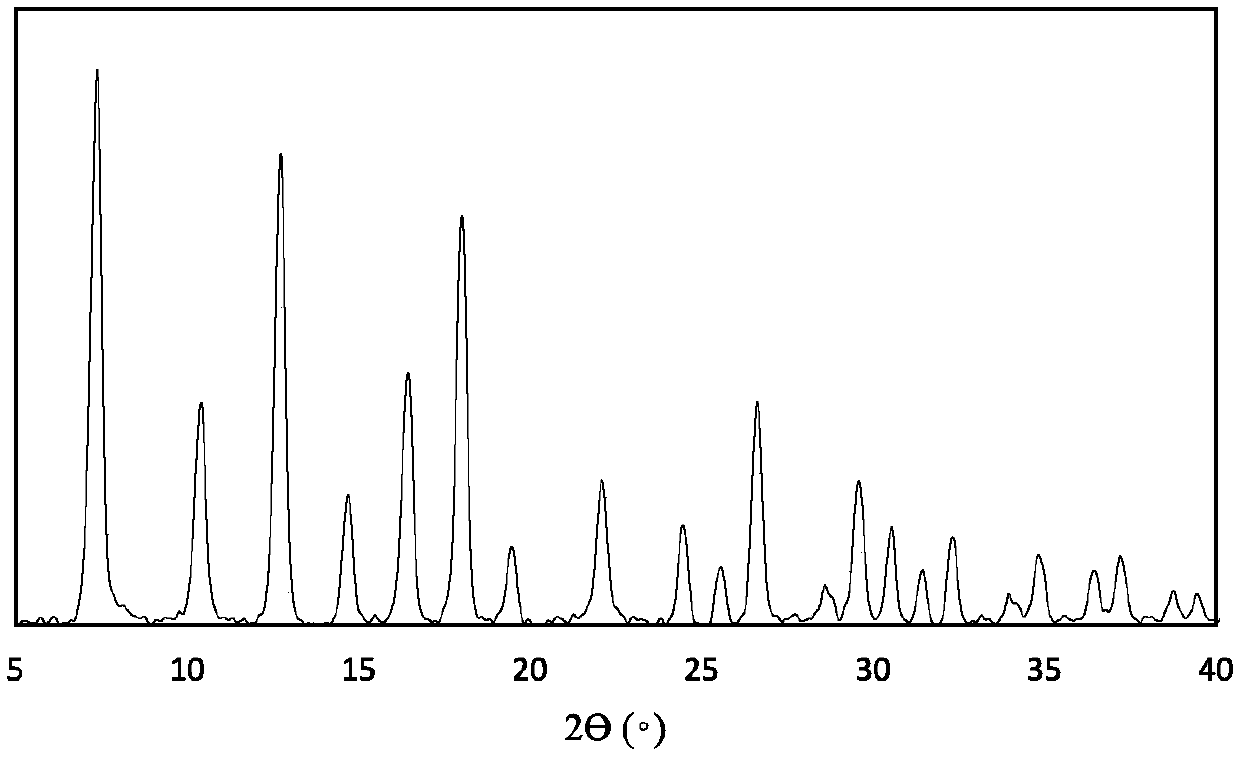
Anticancer drug complex, and preparation method and application thereof
A technology of anti-cancer drugs and complexes, applied in the biological field, can solve the problems of reduced curative effect of chemotherapy drugs, multi-drug resistance of tumors, and multiple targets, and achieve the goal of enhancing targeting performance, good reproducibility, and reducing toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Follow the steps below to synthesize anticancer drug complexes
[0047] According to the molar ratio of 1:2:0.001, zinc acetate dihydrate, 2-imidazole formaldehyde and doxorubicin were successively weighed and placed in a dry hammer bottle, dissolved in an equal volume of N,N-dimethylformamide to prepare Solution, wherein the concentration of zinc acetate dihydrate is 0.2 moles per liter. After the dissolution is completed, take 2 milliliters and mix each, avoid light, and shake at room temperature at 100rpm on a shaker for 1h. After centrifuging to remove the supernatant, the product is vacuum-dried. ZIF-90@doxorubicin.
[0048] Weigh [Arg 6 ,Pro 34 ]pNPY and ZIF-90@doxorubicin were placed in a dry Erlenmeyer flask, then an equal volume of methanol was added, ultrasonicated to form a transparent solution, mixed and stirred in the dark for 48 hours, and the mixed solution of methanol and ethanol with a volume ratio of 20:1 was used to High-speed centrifugal washing, ...
Embodiment 2
[0050] Follow the steps below to synthesize anticancer drug complexes
[0051] According to the molar ratio of 1:1:0.005, zinc acetate, 2-imidazole formaldehyde and cisplatin were successively weighed and placed in a dry hammer bottle, dissolved in an equal volume of N,N-dimethylformamide to prepare a solution, wherein , the concentration of zinc acetate is 0.2 moles per liter. After the dissolution is completed, take 2 ml each to mix, avoid light, and shake at room temperature at 100 rpm on a shaker for 1 hour. After the supernatant is removed by centrifugation, the vacuum-dried product is ZIF-90@通 platinum.
[0052] According to the mass ratio of 1:100, weigh [Arg 6 ,Pro 34 ]pNPY and ZIF-90@cisplatin in a dry Erlenmeyer flask, add an equal volume of methanol, sonicate to form a transparent solution, mix and stir for 12 hours in the dark, and use a mixed solution with a volume ratio of methanol and ethanol of 20:1 for ultra-high speed After centrifugal washing and vacuum dr...
Embodiment 3
[0054] Follow the steps below to synthesize anticancer drug complexes
[0055] According to the molar ratio of 1:5:0.05, zinc acetate hexahydrate, 2-imidazole formaldehyde and vincristine were successively weighed and placed in a dry hammer bottle, dissolved in an equal volume of N,N-dimethylformamide to prepare solution, wherein the concentration of zinc acetate hexahydrate is 0.2 moles per liter, after the dissolution is completed, each take 2 milliliters to mix, avoid light, at room temperature, shake at 100rpm on a shaker for 1h, centrifuge to remove the supernatant and then vacuum dry the product. ZIF-90@vincristine.
[0056] Weigh [Phe 6 ,Pro 34 ]pNPY and ZIF-90@vincristine were placed in a dry Erlenmeyer flask, then an equal volume of methanol was added, ultrasonicated to form a transparent solution, mixed and stirred in the dark for 48 hours, and the mixed solution with a volume ratio of methanol and ethanol of 20:1 was used to High-speed centrifugal washing, vacuum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com