Carbonyl reductase mutant with improved thermal stability
A mutant and reductase technology, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of poor thermal stability of carbonyl reductase ChKRED12, and achieve the effects of flexibility, improved thermal stability, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
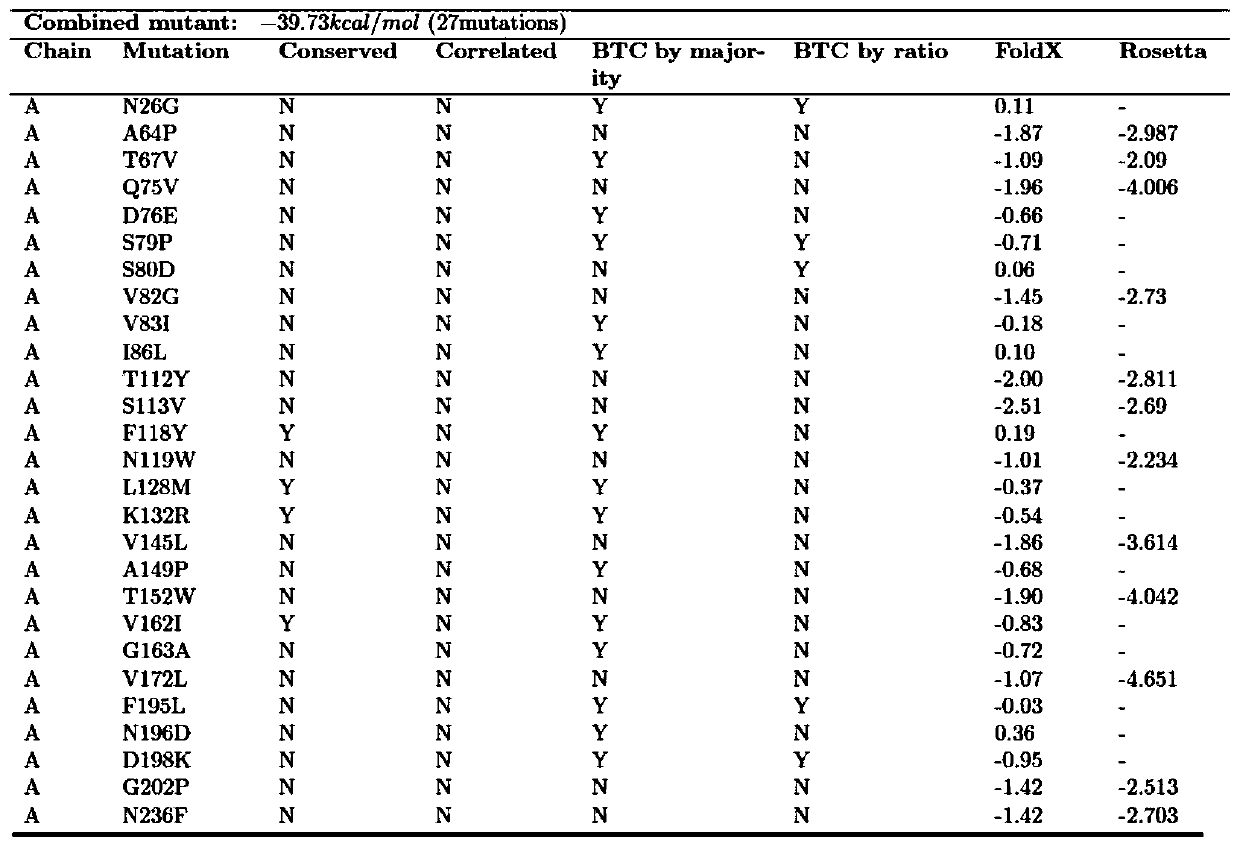
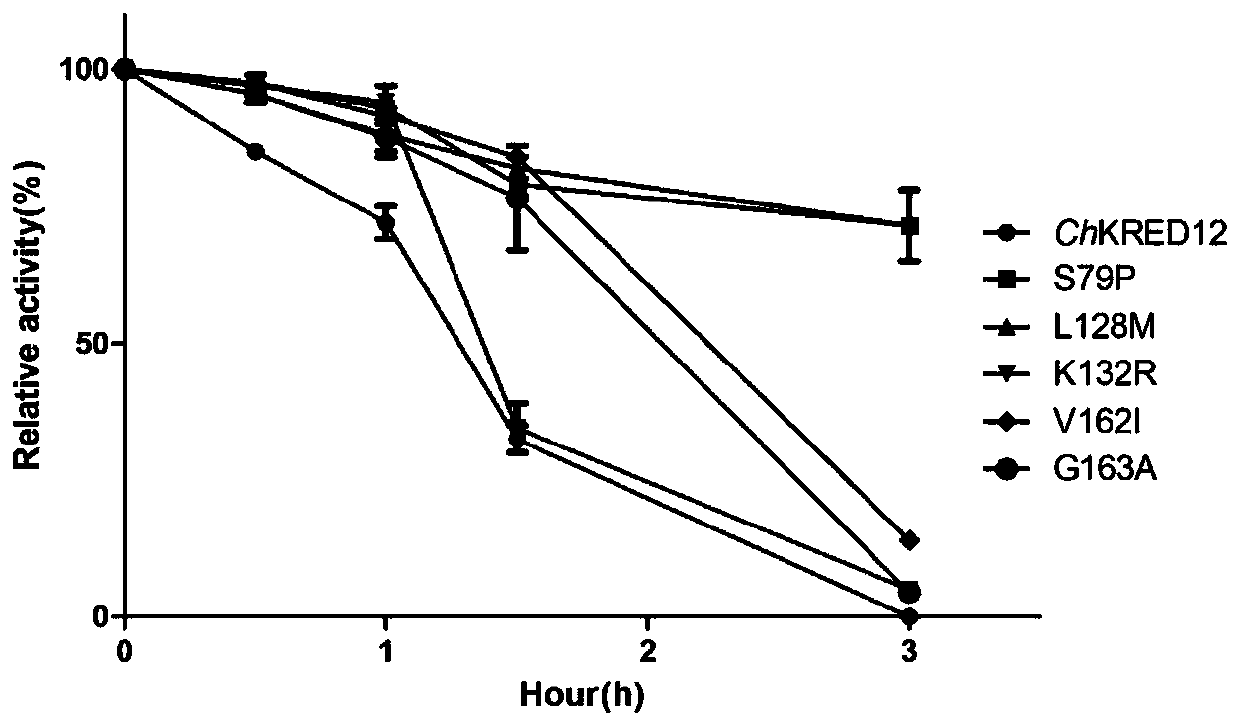
[0035] Example 1 Construction of 4 single point mutants
[0036] The results of the prediction of potential heat-resistant sites by the rational design method are shown in the appendix of the manual. figure 1. The single point mutants S79P, L128M, V162I and G163A were all constructed using the codon-optimized gene SEQ ID NO.1 of carbonyl reductase ChKRED12 as a template, and the primers used were:
[0037] S79P-F:5′–GGATGCGAAT CCG AGCGCCGTGG–3′
[0038] S79P-R:5′–CCACGGCGCT C G ATTCGCATCC–3′
[0039] L128M-F: 5′–CTTTATTCCAGAAA ATG CTGCGTAA C–3′
[0040] L128M-R:5′–GTTACGCAG CAT TTTCTGGATAAA G–3′
[0041] V162I-F:5′–GGCGCGCTG ATT GGGGCGACC–3′
[0042] V162I-R:5′–GGTCGCCCC AAT CAGCGCGCC–3′
[0043] G163A-F: 5′–GGCGCGCTGGTG GCA GCGACCAAAGC–3′
[0044] G163A-R:5′–GCTTTGGTCGC TGC CACCAGCGCGCC–3′
[0045] The PCR conditions are: 5×HF Buffer 10μL, MgCl 2 (1mM) 1μL, primer (50ng / μL) 1.5μL each, dNTP (2.5mM) 4μL, Phu (1U) 1μL, plasmid 50ng, ultrapure water to m...
Embodiment 2
[0046] Embodiment 2 crude enzyme liquid preparation and the mensuration of enzyme activity
[0047] 2.1 Preparation of crude enzyme solution
[0048] Each mutant plasmid in Example 1 was chemically transformed into E. coli expression strain BL21 (DE3), coated with an LB plate containing kanamycin (50 μg / mL), cultured overnight at 37 ° C, and single clones were picked in 10 mL In LB liquid medium containing kanamycin (50 μg / mL), culture overnight at 37° C., 180 rpm. Inoculate 1% inoculum in 200 mL of TB medium containing kanamycin (50 μg / mL), culture at 37 °C for 3 h, add final concentration of 0.5 mM IPTG, induce at 30 °C for 18 h, centrifuge at 5000 rpm and 4 °C for 10 min , discard the supernatant, wash the cells twice with normal saline, then add 15 mL of 0.1M potassium phosphate buffer (pH 8.0) to resuspend the cells, and disrupt the cells with ultrasonic waves (working conditions: working time 3s, intermittent time 3s , working times 99, power 200W), cell disruption sol...
Embodiment 3
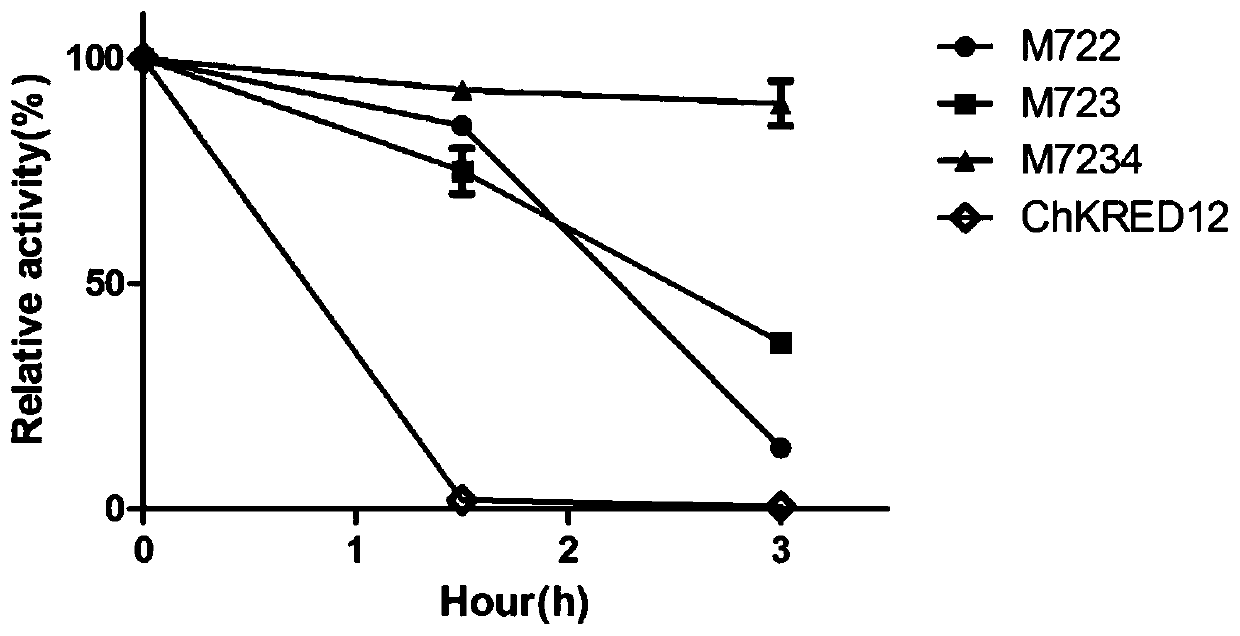
[0060] Example 3 Construction of multi-site combination mutants
[0061] 3.1 Construction of mutant M722
[0062] The site-directed mutagenesis method mutated the 128th leucine of the mutant S79P to methionine (the DNA sequence changed from TTA to ATG), and the 162nd valine to isoleucine (the DNA sequence changed from GTG to ATG). For ATT), construct mutant M722, the primers used are as follows:
[0063] L128M-F: 5′–CTTTATTCCAGAAA ATG CTGCGTAA C–3′
[0064] L128M-R:5′–GTTACGCAG CAT TTTCTGGATAAA G–3′
[0065] V162I-F:5′–GGCGCGCTG ATT GGGGCGACC–3′
[0066] V162I-R:5′–GGTCGCCCC AAT CAGCGCGCC–3′
[0067] The PCR conditions and operations were the same as in Example 1, and a new mutant M722 was obtained.
[0068] 3.2 Construction of mutant M723
[0069] The site-directed mutagenesis method mutated the 128th leucine of the mutant S79P to methionine (the DNA sequence changed from TTA to ATG), and the 163rd glycine to alanine (the DNA sequence changed from GGG to GCA) ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com