Vinylidene fluoride copolymer suitable for binder and its preparation method and application
A vinylidene fluoride-like copolymer technology, which is applied in the field of vinylidene fluoride-like copolymers and its preparation, can solve the problem of high raw material cost and limitations of PVDF-based binders, and PVDF-based binders cannot be used with strong alkalinity environmental issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The second aspect of the present invention provides a method for preparing a vinylidene fluoride copolymer, the method comprising: in the presence of a free radical initiator, in an organic solvent, the monomer represented by formula (1-a), the formula The monomer shown in (2-a) and the monomer shown in formula (3-a) carry out copolymerization reaction, wherein,
[0047] Formula (1-a) Formula (2-a) CF 2 =CH 2 , formula (3-a)
[0048] Wherein, Z is each independently selected from a single bond, -(C m h 2m )-,-(C m f 2m )-, -(CH 2 CH 2 O) m -, -(OCH 2 CH 2 ) m -, -(CO)-O- or -O-(CO)-, k is each independently an integer of 1-5, and m is each independently an integer of 1-20;
[0049] R f for-C h f 2h+1 , h is an integer of 0-10; R f1 , R f2 and R f3 each independently -C i h 2i+1 or -C i f 2i+1 , i is an integer of 0-10;
[0050] Cationic Y + for H + , Li + 、Na + 、K + , Rb + 、Cs + , Mg 2+ , Ca 2+ 、Sr 2+ 、Ba 2+ 、Al 3+ , the cation sho...
preparation example 1
[0092]
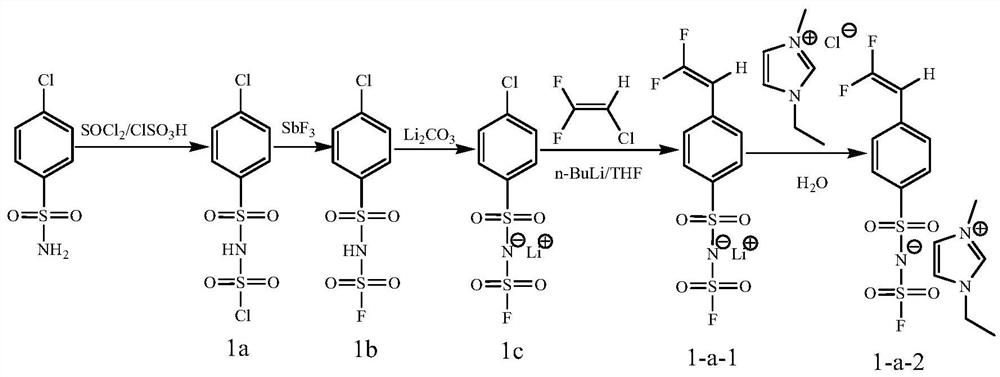
[0093] Prepare the monomer according to the above reaction formula, specifically:
[0094] (1) Take 1.9164g (10mmol) of p-chlorobenzenesulfonamide, 2.3794g (20mmol) of thionyl chloride, and 1.3982g (12mmol) of chlorosulfonic acid at 100°C for 12h to obtain compound 1a (2.6113g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.87(d, 2×1H), 7.55(d, 2×1H), 2.0(s, 1H).
[0095] (2) Take 2.9014g (10mmol) of compound 1a and 2.1451g (12mmol) of SbF 3 Reacted at 60°C for 12h to obtain compound 1b (2.4632g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.87(d, 2×1H), 7.55(d, 2×1H), 2.0(s, 1H).
[0096] (3) Take 2.7369g (10mmol) of compound 1b and 0.7389g (10mmol) of Li 2 CO 3 Reacted at 25°C for 2h to obtain compound 1c (2.7962g, yield 100%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.87(d, 2×1H), 7.55(d, 2×1H), 2.0(s, 1H).
[0097] (4) While cooling with an ice-salt bath, 2.7962 g (10 mmol) of compound 1c was taken, and 10 mL of a tetrahydrofuran solution of 1.6015 g (25...
Embodiment 1
[0100] This example is used to illustrate the vinylidene fluoride copolymer of the present invention and its preparation method.
[0101] Take 6.144g (20mmol) of the compound represented by the formula (1-a-1), 0.1mmol of di-tert-butyl peroxide and 20mL of acetonitrile and mix them uniformly. While heating and stirring at 70° C., a mixed gas (about 20 mmol) of vinylidene fluoride and hexafluoropropylene with a molar ratio of 99:1 was introduced. After the gas feeding process is completed, continue to react for 24h; filter the obtained product, dissolve the solid with 10mL of ethanol, then add 50mL of ether for recrystallization, repeat the recrystallization three times, and then vacuum dry the obtained solid to obtain a white powder The vinylidene fluoride copolymer P1;
[0102] Wherein, the weight average molecular weight of the vinylidene fluoride copolymer is 300,000g / mol, the molecular weight distribution index is 1.3, the structural unit shown in formula (1-1), the struc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com