Method for recovery and reusing of etching liquid containing copper ions and nitrate
A copper ion and etching solution technology, applied in the improvement of process efficiency, instruments, optics, etc., can solve the problems such as the lack of recycling of etching solution, the inability to obtain large-area copper blocks, etc., to achieve easy automatic control, reduce Concentration fluctuation, the effect of huge economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
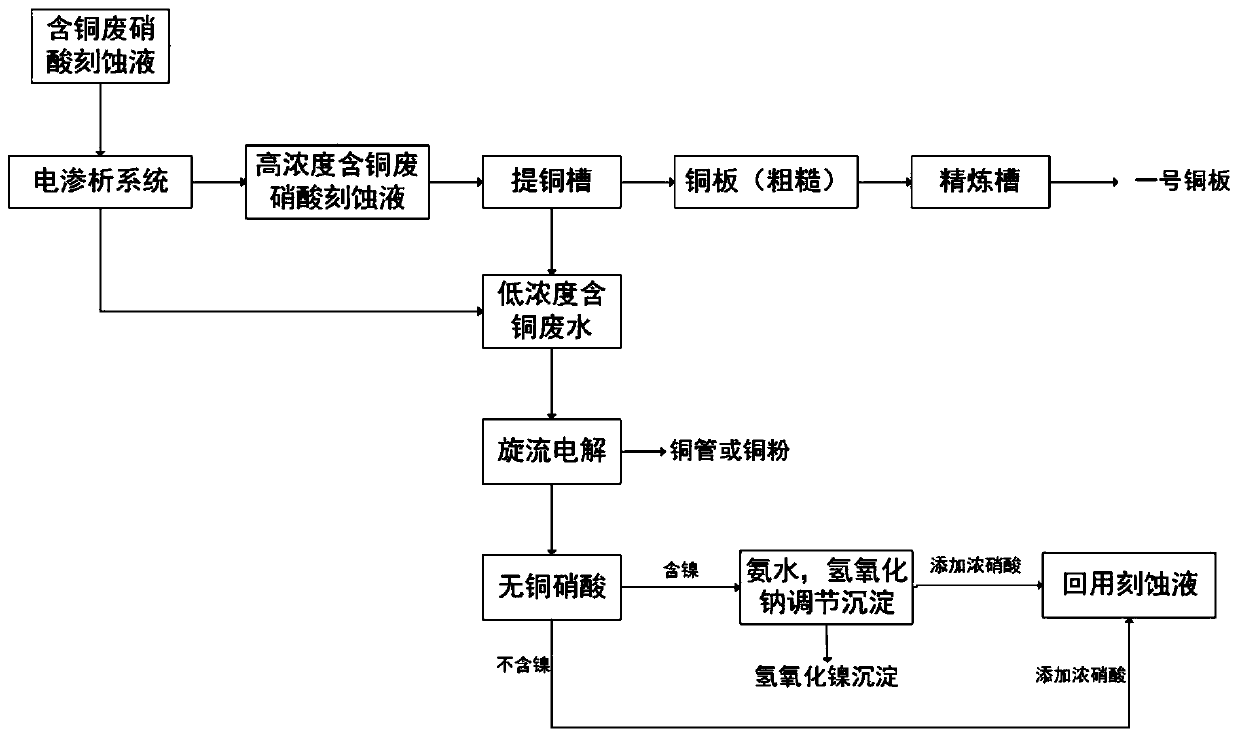
[0047] A method for reclaiming and reusing etching solution containing copper ions, nickel ions and nitrates, comprising the following steps:
[0048] Step A: Collect copper-containing waste nitric acid etching solution, and process the etching solution through an electrodialysis device;
[0049] Step B: the high-concentration copper-containing etching solution produced in step A enters the copper extraction tank for cyclic electrolysis;
[0050] Step C: using the thick copper plate produced in step B as an anode, and electrolyzing it in a refining tank to form No. 1 copper plate;
[0051] Step D: collecting the low-concentration copper-containing wastewater produced in steps A and B, and performing cyclone electrolysis on the wastewater to obtain copper pipes and copper powder;
[0052] Step E: if the waste liquid contains nickel, add ammonia water, sodium hydroxide and other agents to the waste liquid to adjust the pH to precipitate nickel ions;
[0053] Step F: add a cert...
Embodiment 2
[0062] A method for reclaiming and reusing etching solution containing copper ions and nitrates, comprising the following steps:
[0063] Step A: Collect waste nitric acid etching solution containing copper, and process the etching solution through an electrodialysis device;
[0064] Step B: the high-concentration copper-containing etching solution produced in step A enters the copper extraction tank for cyclic electrolysis;
[0065] Step C: using the thick copper plate produced in step B as an anode, and electrolyzing it in a refining tank to form No. 1 copper plate;
[0066] Step D: collecting the low-concentration copper-containing wastewater produced in steps A and B, and performing cyclone electrolysis on the wastewater to obtain copper pipes and copper powder;
[0067] If the waste liquid contains nickel, add ammonia water, sodium hydroxide and other agents to the waste liquid to adjust the pH to precipitate nickel ions;
[0068] Step E: if the waste liquid contains ni...
Embodiment 3
[0077] A method for reclaiming and reusing etching solution containing copper ions and nitrates, comprising the following steps:
[0078] Step A: Collect waste nitric acid etching solution containing copper, and process the etching solution through an electrodialysis device;
[0079] Step B: the high-concentration copper-containing etching solution produced in step A enters the copper extraction tank for cyclic electrolysis;
[0080] Step C: using the thick copper plate produced in step B as an anode, and electrolyzing it in a refining tank to form No. 1 copper plate;
[0081] Step D: collecting the low-concentration copper-containing wastewater produced in steps A and B, and performing cyclone electrolysis on the wastewater to obtain copper pipes and copper powder;
[0082] If the waste liquid contains nickel, add ammonia water, sodium hydroxide and other agents to the waste liquid to adjust the pH to precipitate nickel ions;
[0083] Step E: if the waste liquid contains ni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com