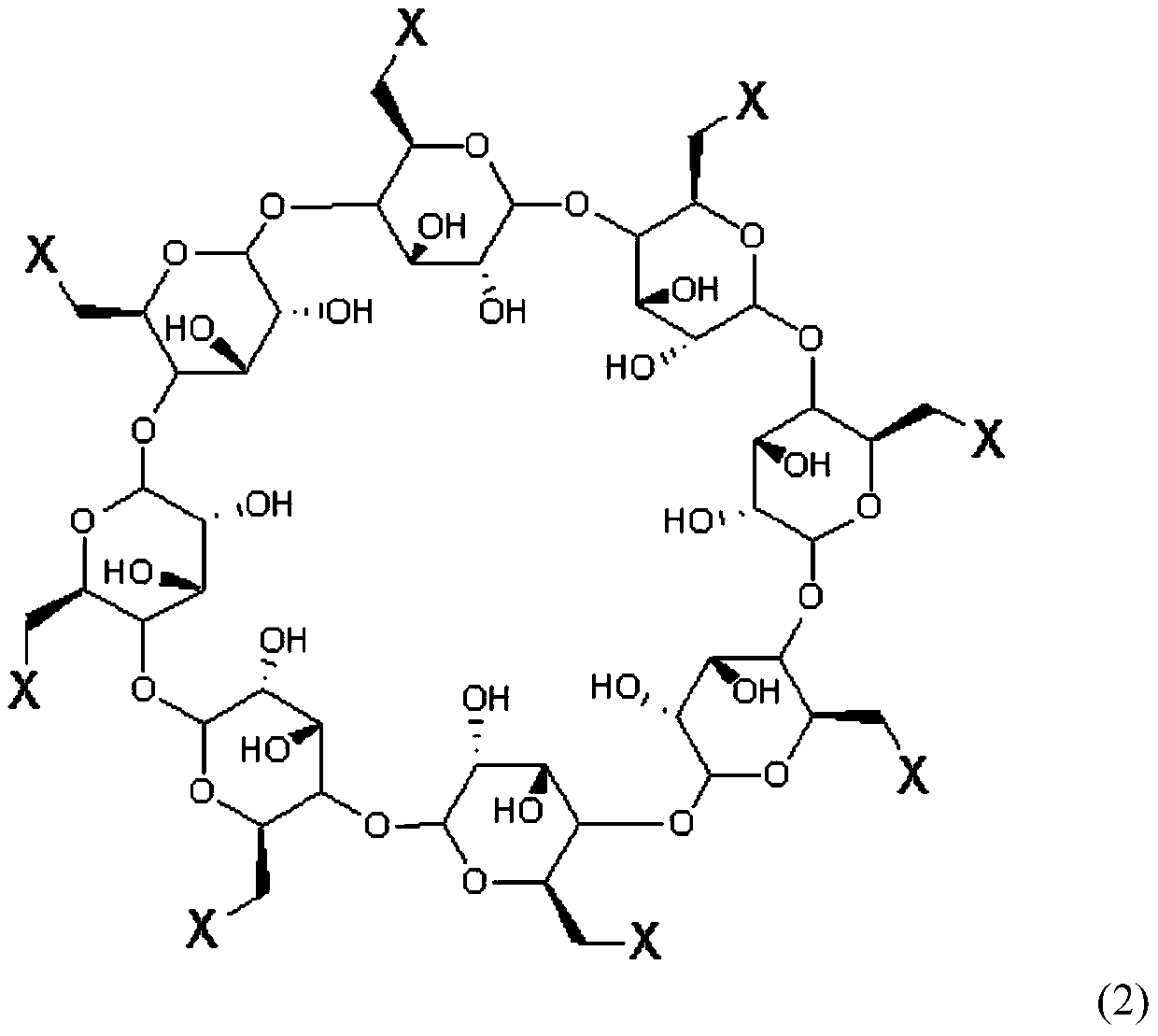
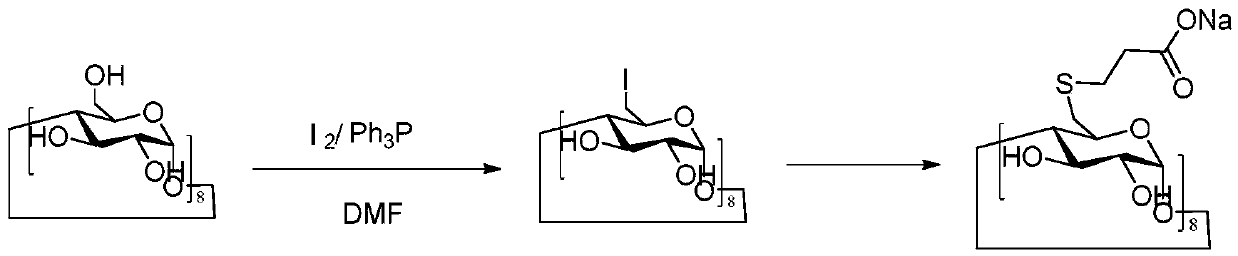
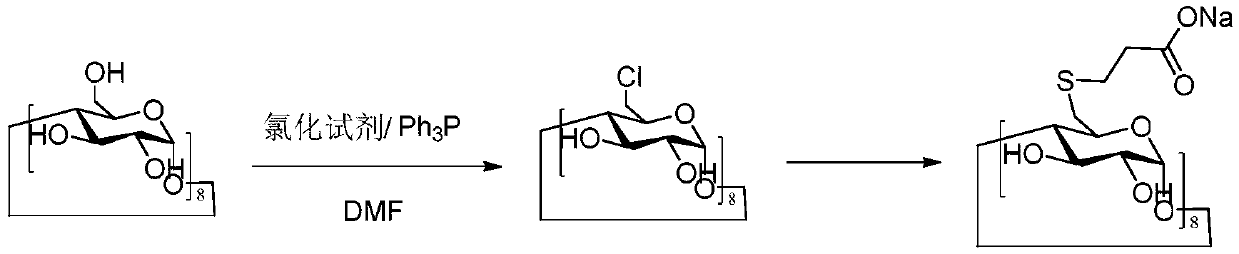
Method for preparing sugammadex sodium and sugammadex sodium intermediate
A technology of sugammadex sodium and intermediates, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as high cost, difficult operation, and large pollution, and achieve the effects of environmentally friendly synthesis methods, reduced production costs, and convenient production operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of Sugammadex Sodium
[0057] The first step: the key intermediate 6-perdeoxy-6-perbromo-γ-cyclodextrin
[0058] Under the protection of nitrogen, 4 kg of dimethylformamide, 1.5 kg of γ-cyclodextrin (dried at 105° C.) and 5.0 kg of triphenylphosphine solution were sequentially added into a 50 L reactor. Cool the reaction system to 0-5°C, add 3.5kg of dibromohydantoin in DMF dropwise, and control the temperature during the dropwise addition to ≤60°C. After the dropwise addition, raise the temperature of the reaction system to 75-90°C, and stir for 4 hours.
[0059] After the reaction is complete, cool the system down to room temperature, transfer it to a 100L enamel reaction kettle, add 2.0kg of methanol, cool the reaction system down to room temperature, add a pre-configured sodium hydroxide aqueous solution dropwise to adjust the pH to 8-10; then add water dropwise 30kg, after the dropwise addition was completed, the temperature was controlled and stirred ...
Embodiment 2
[0069] Preparation of key intermediate 6-perdeoxy-6-perbromo-γ-cyclodextrin (formula II)
[0070] Under the protection of nitrogen, 400 ml of dimethylformamide, 150 g of γ-cyclodextrin (not dried) and 550 g of triphenylphosphine solution were sequentially added into a 5 L reaction flask. Cool the reaction system to 0-5°C, add 400g of dibromohydantoin in DMF dropwise, and control the temperature during the dropwise addition to ≤60°C. After the dropwise addition, raise the temperature of the reaction system to 75-90°C, and stir for 4 hours.
[0071] After the reaction is complete, cool the system down to room temperature, transfer it to a 5L glass reactor, add 200g of methanol, cool the reaction system down to room temperature, add dropwise a preconfigured aqueous sodium hydroxide solution to adjust the pH to 10; then add dropwise 3.0kg of water, After the dropwise addition was completed, the mixture was stirred at room temperature for 4 hours. Suction filtration, the filter ca...
Embodiment 3
[0073] Preparation of key intermediate 6-perdeoxy-6-perbromo-γ-cyclodextrin (formula II)
[0074] Under the protection of nitrogen, 50 ml of dimethylformamide, 15 g of γ-cyclodextrin (not dried) and 500 g of triphenylphosphine solution were sequentially added into a 500 ml reaction flask. Cool the reaction system to 0-5°C, add dropwise a solution of 350g of dibromohydantoin in dimethylformamide, and control the temperature during the dropwise addition to ≤60°C. After the dropwise addition, raise the temperature of the reaction system to 75-90°C, and stir Reaction 4h.
[0075] After the reaction is complete, cool the system to room temperature, transfer it to a 1L reaction bottle, add 30ml of methanol, cool the reaction system to room temperature, add dropwise a pre-configured aqueous sodium hydroxide solution to adjust the pH to 10; then add 300g of water dropwise, and add Complete temperature control and stir at room temperature for 5 h. Suction filtration, the filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com