Sulfide sodium ion solid electrolyte and preparation method thereof
A solid electrolyte and sodium ion technology, used in circuits, electrical components, secondary batteries, etc., can solve the problems of air sensitivity, solid electrolyte failure, and few types, reducing activation energy, improving electrochemical and chemical stability, The effect of improving ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] According to another aspect of the present invention, a kind of preparation method of air stable sulfide sodium ion solid electrolyte is provided, comprising:
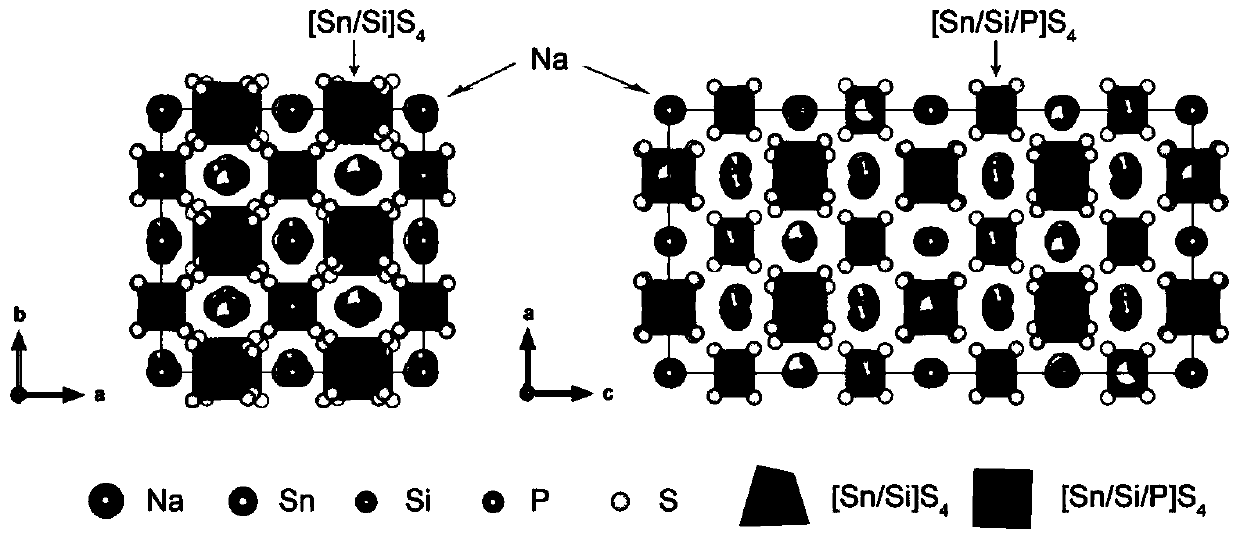
[0033] (1), the Na 2 S, MS, (M=Ge, Si), SnS 2 ,P 2 S,(N=P,Sb),Sb 2 S 3 ,S,NaX,(X=Cl,Br,I) according to Na 4-x-z [Sn 1-y m y ]N x S 4-z x z The mol ratio is uniformly mixed to obtain mixed raw materials;
[0034] (2) Perform solid-phase synthesis of the mixed raw materials under anaerobic conditions at 400°C to 650°C to obtain Na 4-x-z [Sn 1- y m y ]N x S 4-z x z , whose crystal structure satisfies I4 1 / acd space group; anaerobic conditions are vacuum conditions less than 100Pa.
[0035] Wherein, M is at least one of Si or Ge, N is at least one of P or Sb, X is at least one of Cl, Br, and I; 0≤x≤0.6, 0.30≤y≤0.35, 0 ≤ z ≤ 0.1.
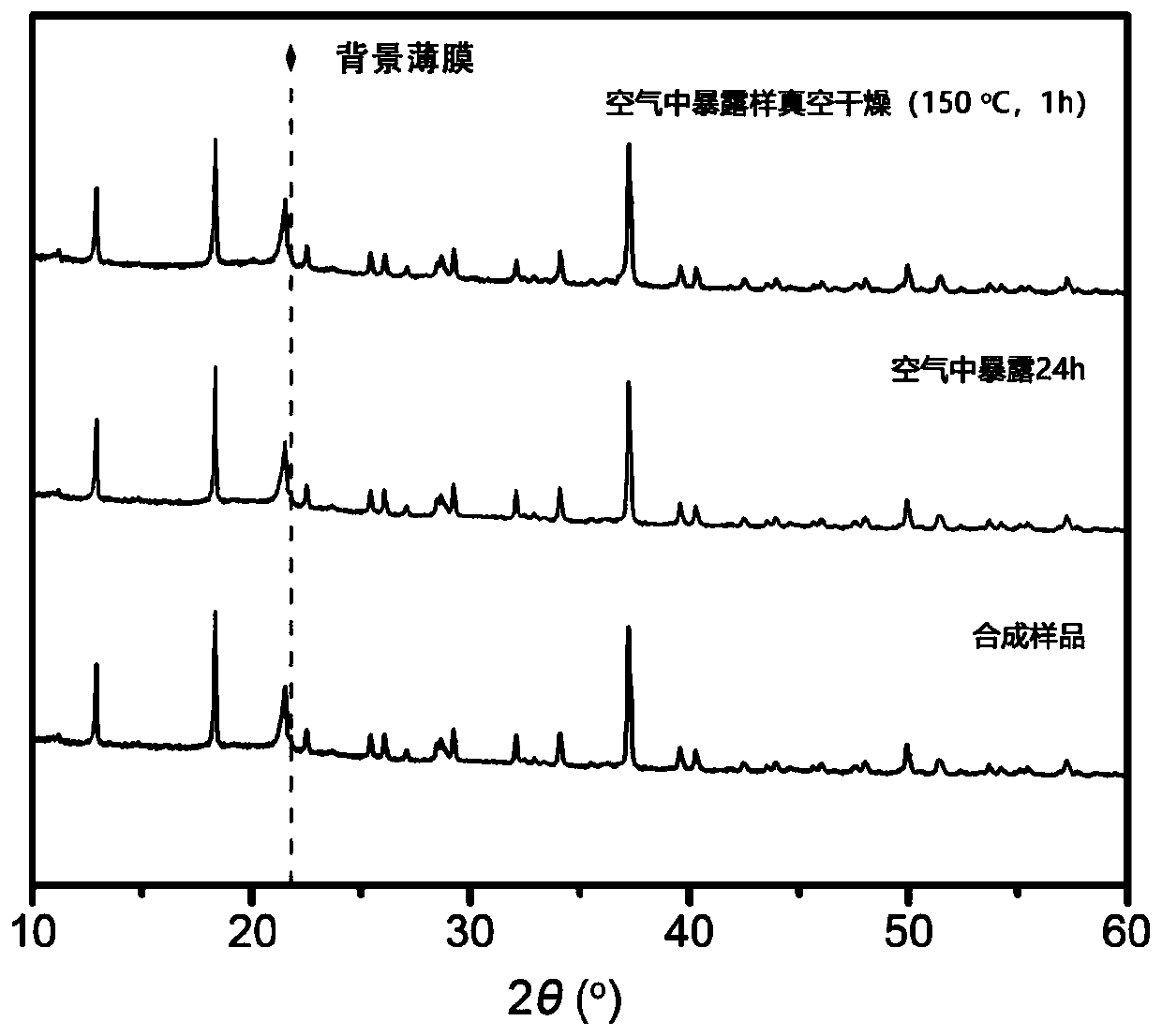
[0036] Further, the solid-phase synthesis time is 8h-24h. High temperature is beneficial to the preparation reaction of sulfide solid electrolytes, increases the crys...
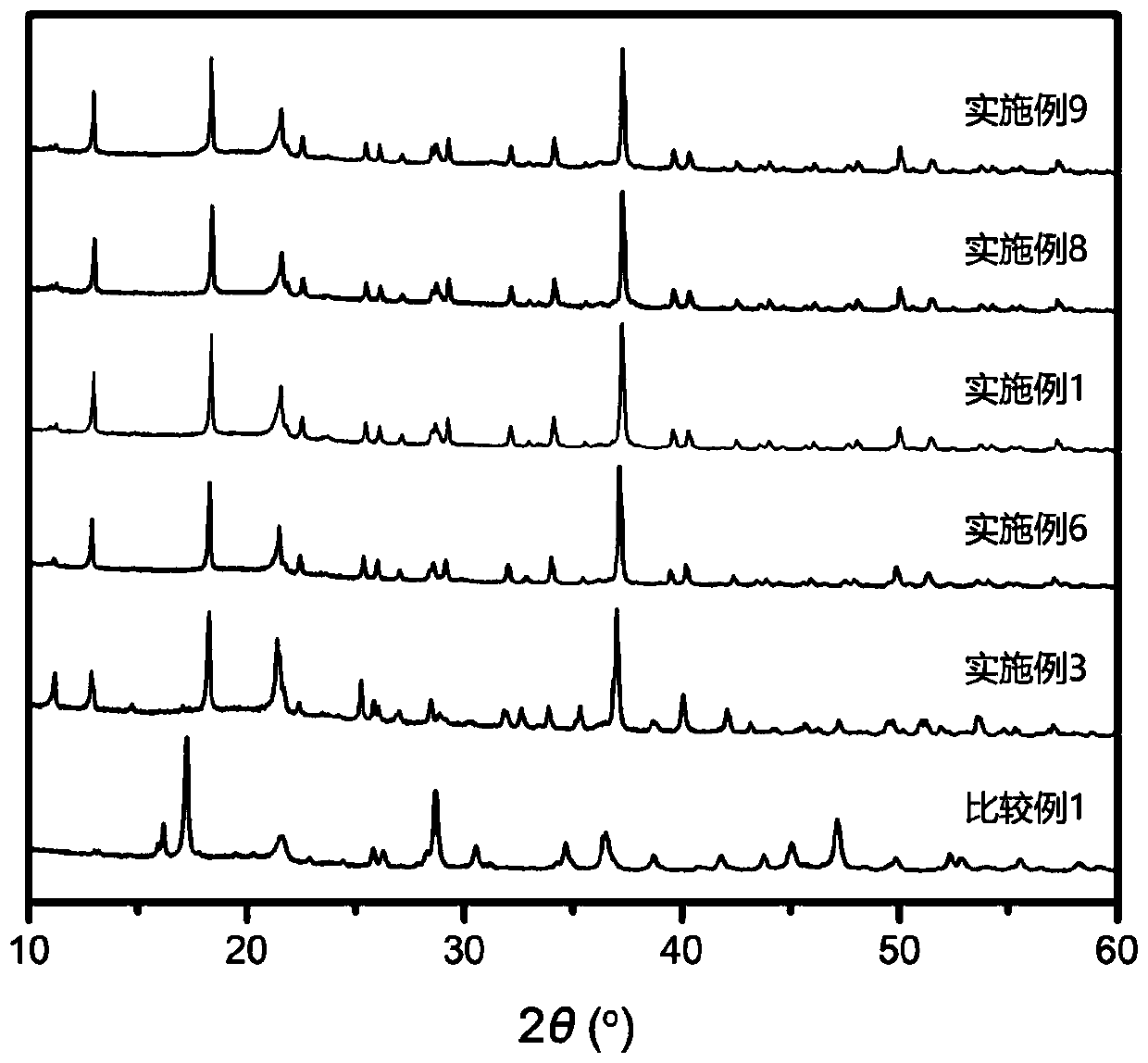
Embodiment 1
[0050] Na in an argon-protected glove box 2 S, P 2 S 5 , SnS 2 、SiS 2 Take Na 3.7 [Sn 0.67 Si 0.33 ] 0.7 P 0.3 S 4 The mol ratio is weighed and mixed as raw materials;
[0051] Put the raw materials and zirconia balls into a zirconia-substrated ball mill tank with a capacity, seal the container, perform ball mill mixing at a speed of 360r / min, and obtain a mixed powder after 18 hours;
[0052]Put the mixed powder in the glove box, take it out, and form it under 150MPa pressure in a powder tablet press, put it into a glass / quartz tube, evacuate it until the vacuum degree is less than 100Pa, seal it, and put it into a muffle furnace. The temperature rise rate of the muffle furnace is 100°C / hour, and then the solid phase reaction is carried out at 550°C for 24 hours, and the temperature is naturally cooled to obtain the product Na 4-x [Sn 1-y m y ]N x S 4 , the product in this example is Na 3.7 [Sn 0.67 Si 0.33 ] 0.7 P 0.3 S 4 .
[0053] In the glove box, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com