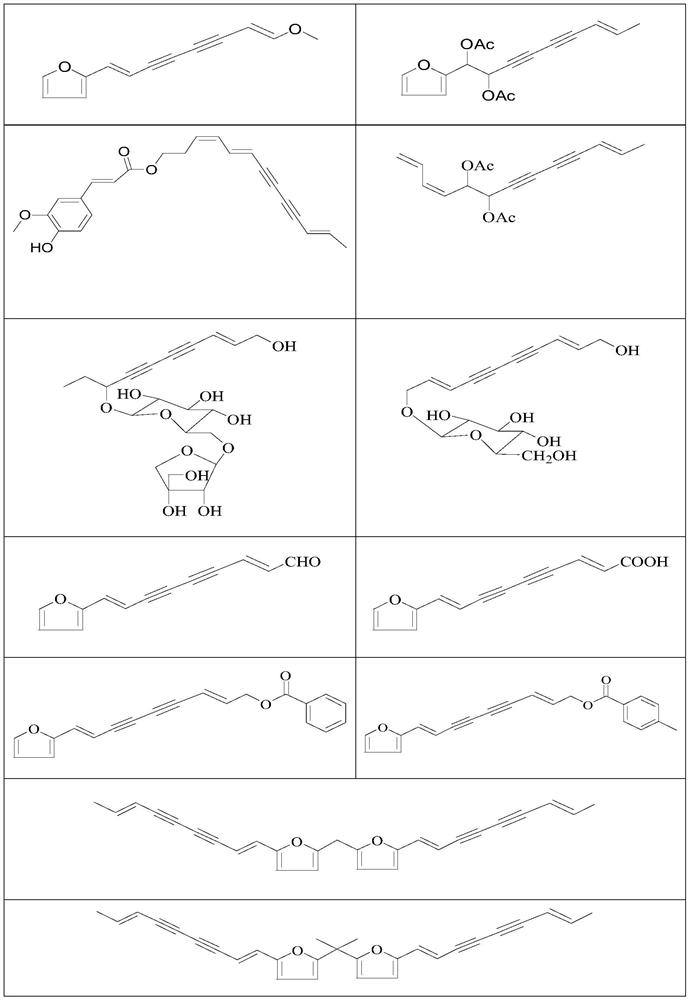
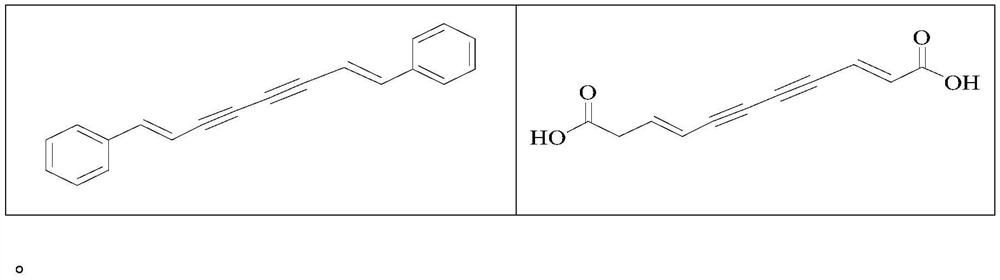
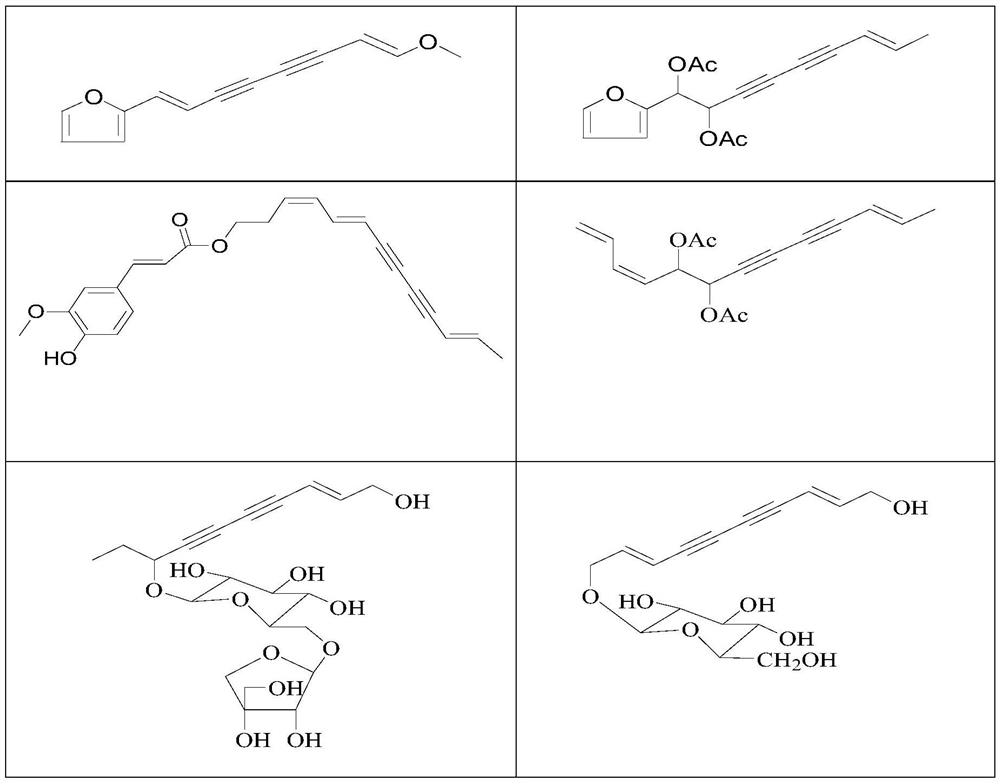
Use of a polyacetylenic compound for reducing uric acid
A compound and uric acid-lowering technology, applied in the field of pharmaceuticals, can solve the problems of weak xanthine oxidase inhibitory effect and inability to lower uric acid gout, etc., and achieve a significant effect of lowering uric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of Compound 1-6
[0066]
[0067] The specific operation steps are as follows:
[0068] Take 100 kg of herb decoction pieces, add 10 times the volume of 70% ethanol aqueous solution to extract 3 times at 80°C, extract for 1.5 hours each time, filter to remove the medicinal residue, and concentrate under reduced pressure to 100L, wherein the solid content is about 15kg, to obtain concentrated solution A .
[0069] The concentrated solution A was separated by a low-pressure D101 column (column diameter 28cm×height 162cm, column volume 100L), and was eluted with an ethanol-water gradient (first eluted 4BV with 30% ethanol-water solution, then eluted 4BV with 95% ethanol-water solution ), collect the eluate of 95% ethanol aqueous solution and concentrate to a solid content of about 3kg to obtain concentrate B.
[0070] The concentrated solution B was separated by LX-20SS column (column diameter 20cm×height 78cm, column volume 25L), and ethanol aq...
Embodiment 2
[0076] Example 2 Preparation of Compound 7-12
[0077]
[0078]
[0079] The synthetic route is as follows:
[0080]
[0081] The reaction conditions are as follows:
[0082] a: SeO 2 , 1,4-dioxane, 90°C;
[0083] b: (1) Ag 2 O,H 2 O / NaOH (40%), 0°C; (2) HCl (36%), pH=5, r.t.;
[0084] c: Benzoic acid, DCC, DMAP, CH 2 Cl 2 , r.t.;
[0085] d: 4-methylbenzoic acid, DCC, DMAP, CH 2 Cl 2 , r.t.;
[0086] e: HCHO, AlCl 3 , HCl, CH 2 Cl 2 ;
[0087] f:CH 3 COOCH 3 , HCl / AcOH (10%), r.t..
[0088] The specific operation steps are as follows:
[0089] Take 10g atractylodes and dissolve in 30mL 1,4-dioxane, add 0.6g SeO to the solution 2 , stirred and reacted at 90°C for 8 hours, then filtered the reaction solution through a 45 μM microporous filter, and then performed silica gel column chromatography with petroleum ether-ethyl acetate at a volume ratio of 20:1 to 5:1 as the mobile phase. After separation and purification, 0.6 g of the compound (yield 6%...
Embodiment 3
[0095] Example 3 Preparation of Compound 13
[0096]
[0097] The synthetic route is as follows:
[0098]
[0099] The specific operation steps are as follows:
[0100] Under nitrogen protection, will contain 2.0mol 1-bromo-2-styrene, 0.02mol PdCl 2 (PPh 3 ) 2 , 0.04mol CuI and 1.5mol Et 3 The 0.2 mol / L THF solution of N was stirred rapidly, and the 0.2 mol / L THF solution containing 1.0 mol 1,4-bis(trimethylsilyl)-1,3-butadiyne was added at room temperature. After reacting for 1 hour, with 100 mL of saturated NH 4 The reaction was quenched with aqueous Cl solution, and then extracted with 3*50 mL ethyl acetate to obtain an ethyl acetate organic layer extract. The ethyl acetate organic layer extract was washed with 50 mL of saturated NaCl aqueous solution, and then washed with Na2SO 4 Dry and concentrate in vacuo to obtain a concentrated solution. The concentrated solution is separated and purified by silica gel column chromatography with petroleum ether-ethyl a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com