3,3-disubstituted indolinone and its derivative, and synthesis method and application thereof
A technology of indolinone and synthetic method, which is applied in 3 fields, can solve the problems of poor selectivity and limited scope of application of substrates, and achieve the effects of easy purification, high atom economy and step economy, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111]
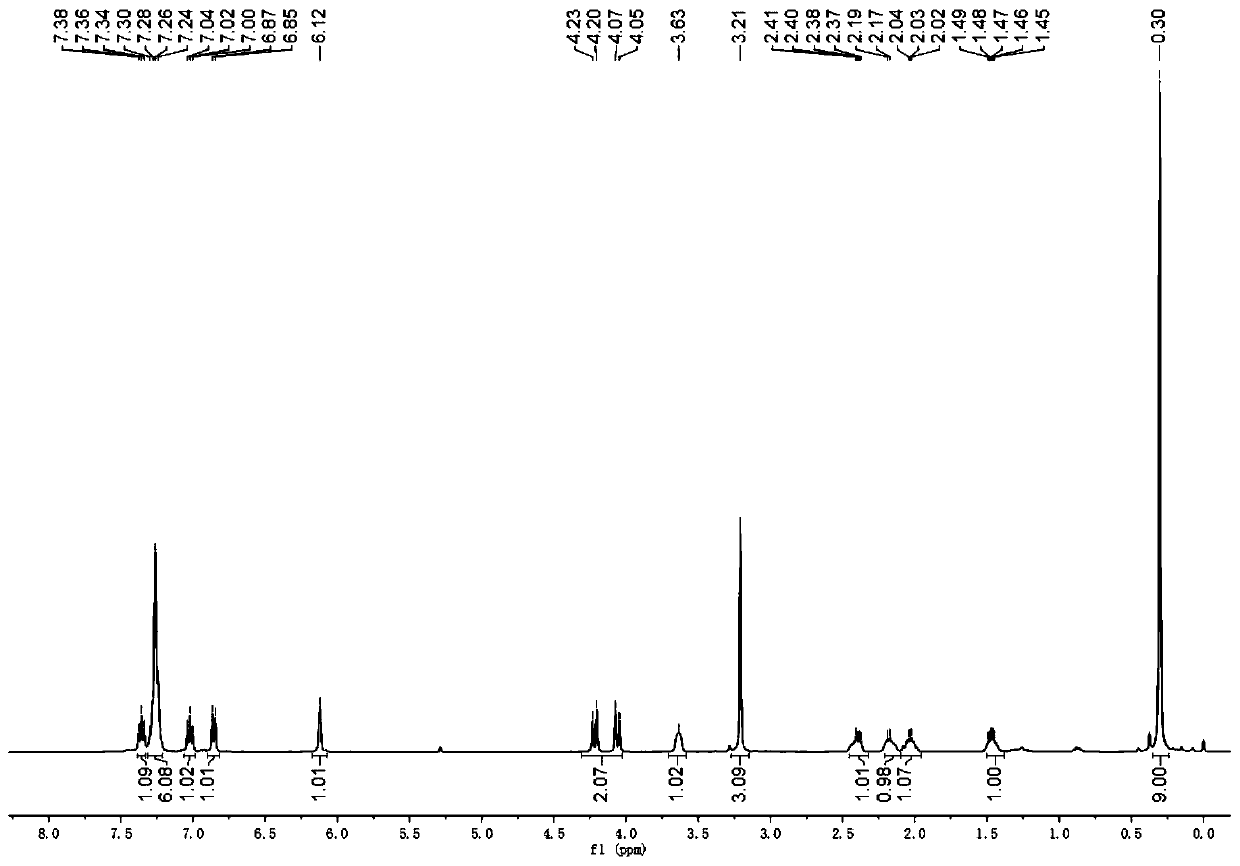
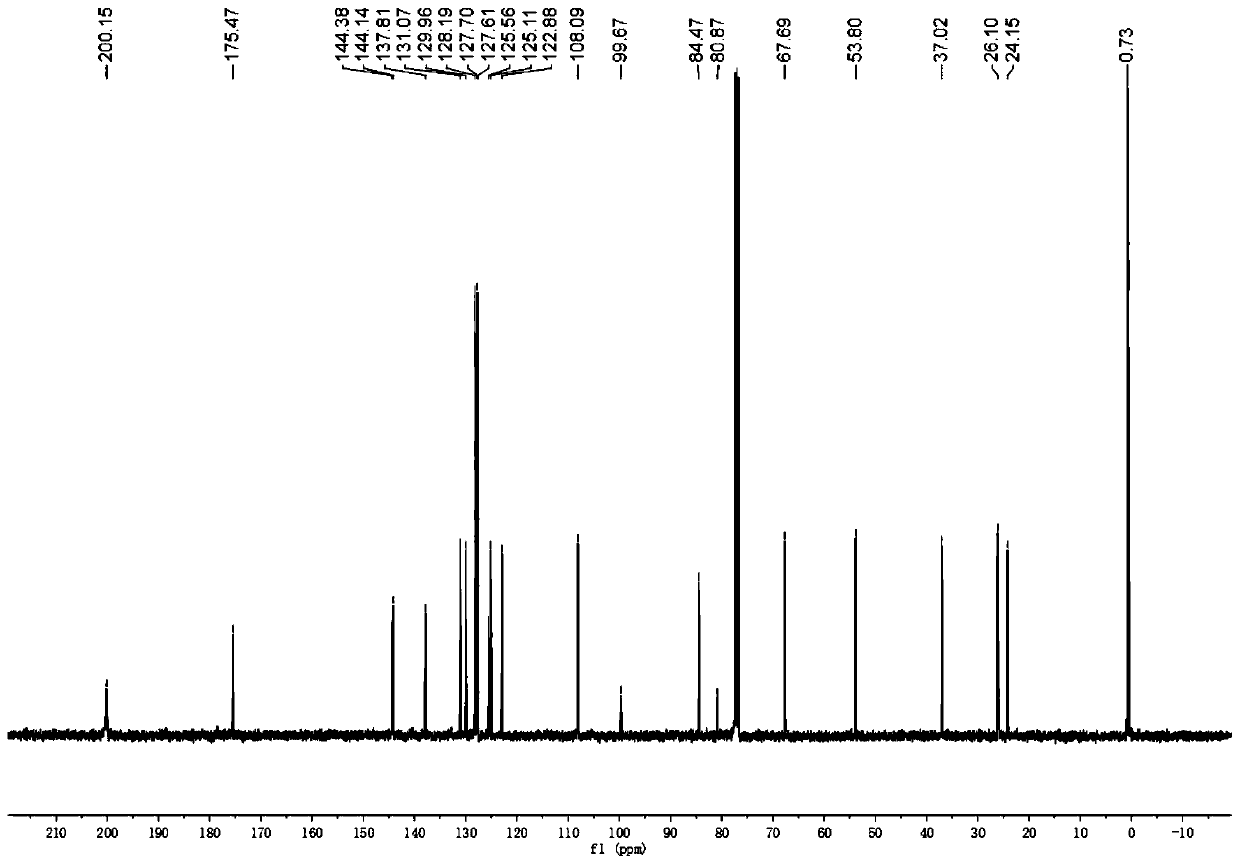
[0112] Under nitrogen protection, silver tetrafluoroborate and rhodium acetate were added to a dry 10ml test tube. After adding 1.5ml of dichloromethane, the temperature was raised to 40°C, propynyl alcohol hexacarbonyl dicobalt 1 (0.1mmol), benzyl alcohol 2 (0.15mmol), 3-diazoindolinone 3 (0.15mmol) were mixed and dissolved In 1 ml of dichloromethane, pour the mixed solution into a test tube within 2 hours using a peristaltic pump, and continue stirring for 0.5 hour after the dropwise addition. After the solvent was removed from the reaction solution under reduced pressure, a sample was taken and dissolved in deuterated chloroform for use 1 The d.r. value was determined by H NMR, and all the solutions were mixed and purified by column chromatography (petroleum ether: ethyl acetate = 70:1-20:1) to obtain pure product 4a. Yield: 90%, anti:syn=43:57.
[0113] Compound syn-4a: 1 H NMR (400MHz, CDCl 3 )δ7.36(t, J=7.7Hz, 1H), 7.31-7.21(m, 6H), 7.02(t, J=7.5Hz, 1H), ...
Embodiment 2
[0116]
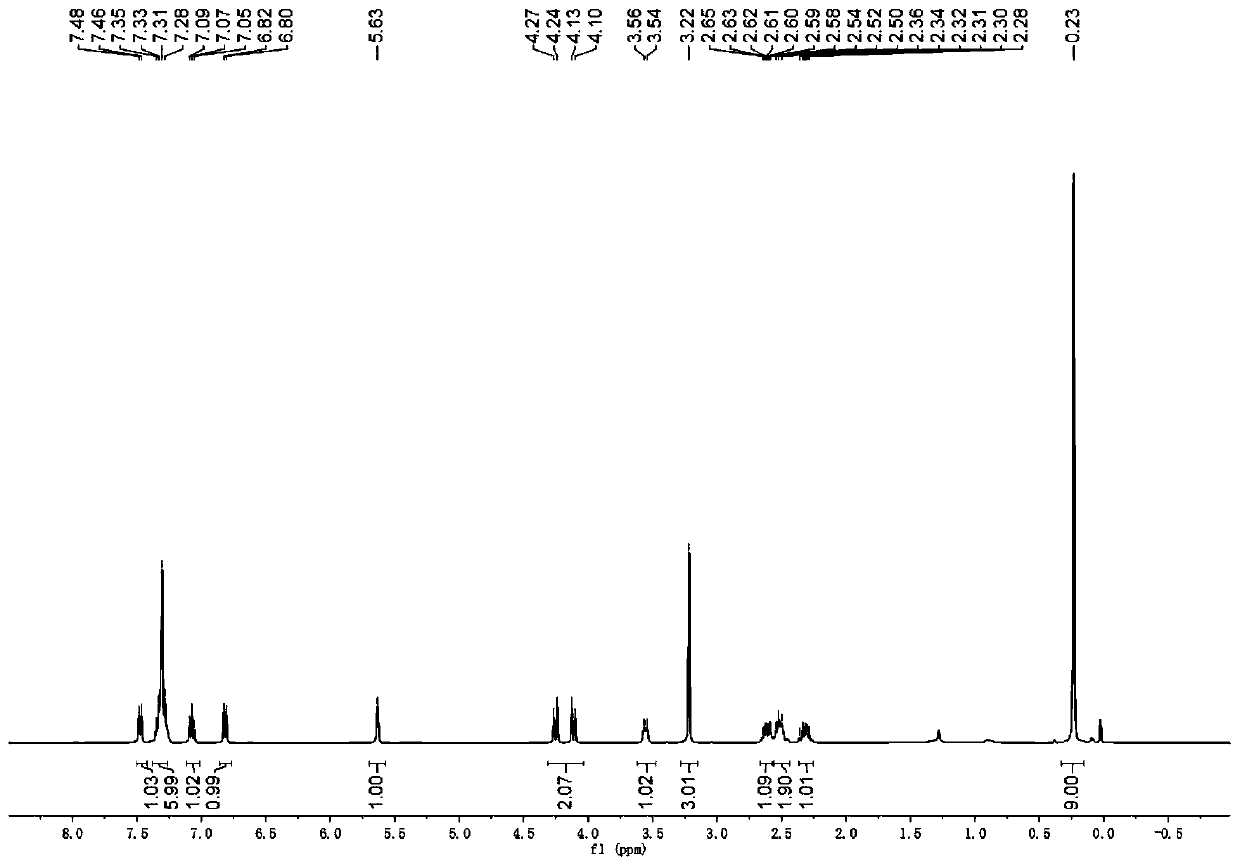
[0117] Under nitrogen protection, silver tetrafluoroborate and rhodium acetate were added to a dry 10ml test tube. After adding 1.5ml of dichloromethane, the temperature was raised to 40°C, propynyl alcohol hexacarbonyl dicobalt 1 (0.1mmol), benzyl alcohol 2 (0.15mmol), 3-diazoindolinone 3 (0.15mmol) were mixed and dissolved In 1 ml of dichloromethane, pour the mixed solution into a test tube within 2 hours using a peristaltic pump, and continue stirring for 0.5 hour after the dropwise addition. After the solvent was removed from the reaction solution under reduced pressure, a sample was taken and dissolved in deuterated chloroform for use 1 The d.r. value was measured by H NMR, and all solutions were mixed and purified by column chromatography (petroleum ether: ethyl acetate = 70:1-20:1) to obtain pure product 4b. Yield: 66%, anti:syn=47:53.
[0118] Compound syn-4b: 1 H NMR (400MHz, CDCl 3 )δ7.36(t, J=7.7Hz, 1H), 7.24(s, 1H), 7.17(d, J=8.4Hz, 2H), 7.03(t, J=7...
Embodiment 3
[0121]
[0122] Under nitrogen protection, silver tetrafluoroborate and rhodium acetate were added to a dry 10ml test tube. After adding 1.5ml of dichloromethane, the temperature was raised to 40°C, propynyl alcohol hexacarbonyl dicobalt 1 (0.1mmol), benzyl alcohol 2 (0.15mmol), 3-diazoindolinone 3 (0.15mmol) were mixed and dissolved In 1 ml of dichloromethane, pour the mixed solution into a test tube within 2 hours using a peristaltic pump, and continue stirring for 0.5 hour after the dropwise addition. After the solvent was removed from the reaction solution under reduced pressure, a sample was taken and dissolved in deuterated chloroform for use 1 The d.r. value was determined by H NMR, and all the solutions were mixed and purified by column chromatography (petroleum ether: ethyl acetate = 70:1-20:1) to obtain pure product 4c. Yield: 77%, anti:syn=43:57.
[0123] Compound syn-4c: 1 H NMR (400MHz, CDCl 3 )δ7.38(dd, J=16.8, 8.0Hz, 3H), 7.22(d, J=7.2Hz, 1H), 7.13(d, J=8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com