Synthetic method of iohexol impurity F and application thereof in synthesis of iohexol impurity G, impurity H and impurity M
A synthesis method and technology of iohexol are applied in the field of medicine to achieve the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
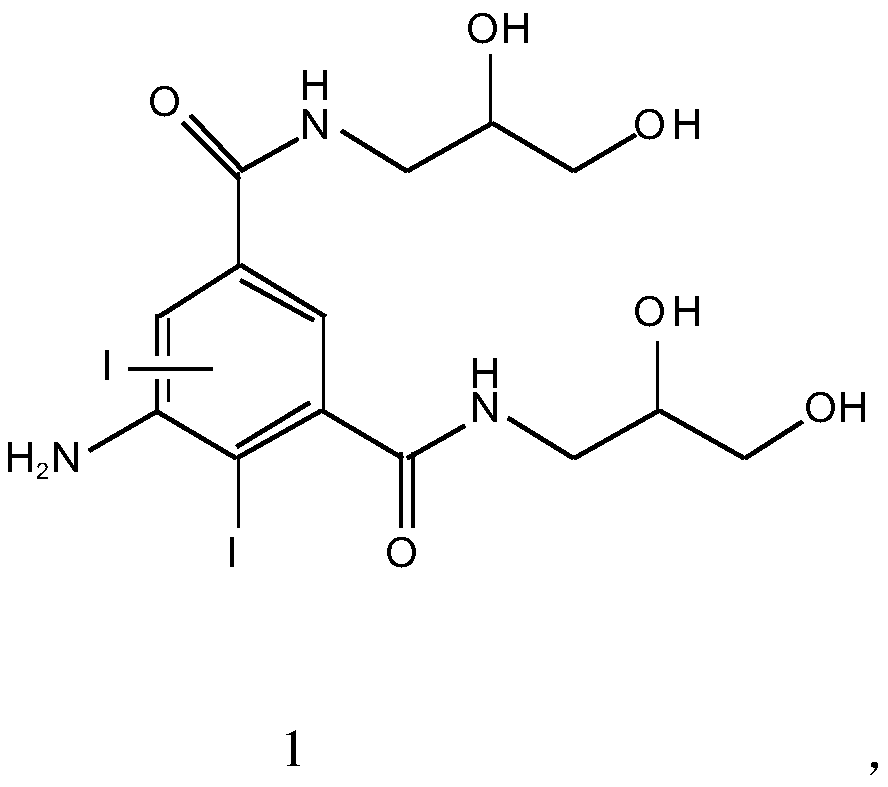
[0034] Embodiment 1: the synthesis of iohexol impurity F (compound of formula 1)
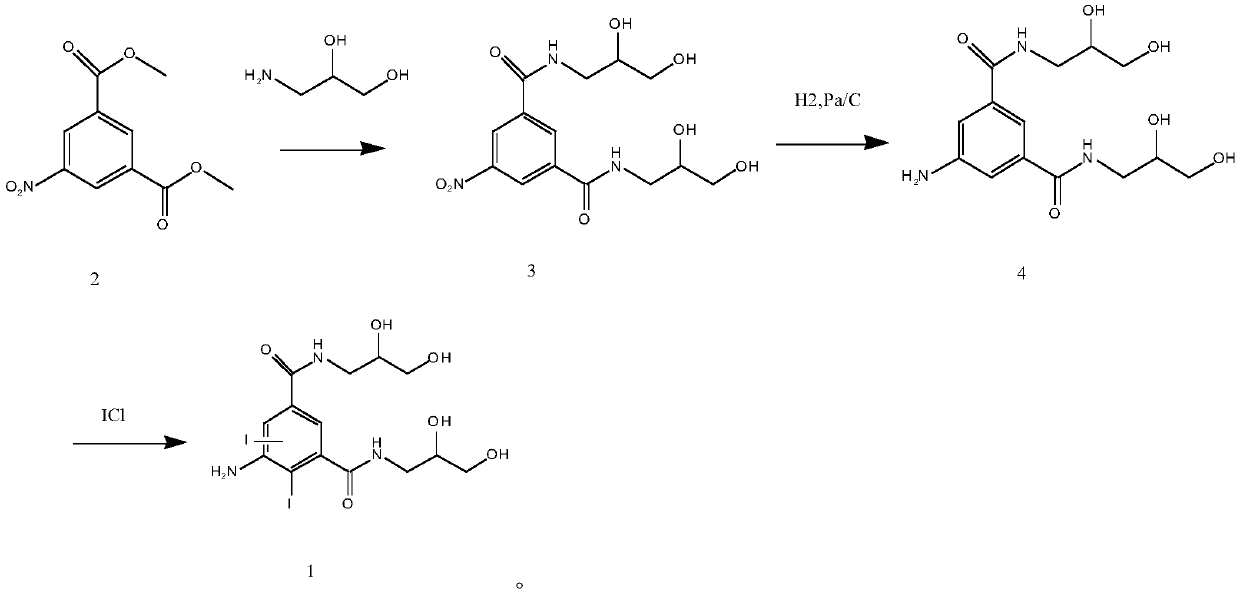
[0035] 1. Synthesis of 5-nitro-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide (compound of formula 3)
[0036] Prepare raw materials according to the molar ratio of dimethyl 5-nitro-1,3-phthalate to 2,3-dihydroxypropylamine at 1:1.2. Put 50g of dimethyl 5-nitro-1,3-phthalate into a 500ml reaction bottle, add 80mL of methanol, stir evenly, then add 22.8g of 2,3-dihydroxypropylamine dropwise, and add 5g of Sodium methoxide, keep the temperature at 20-50°C and react for 20-40 hours. HPLC detects that the raw material is ≤0.1% and the reaction is complete. Cool down to room temperature and adjust the pH to 6 with hydrochloric acid. Add 250mL of water and mix to obtain about 450mL of transesterification reaction solution for the following One-step hydrogenation reduction reaction.
[0037] 2. Synthesis of 5-amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarbox...
Embodiment 2
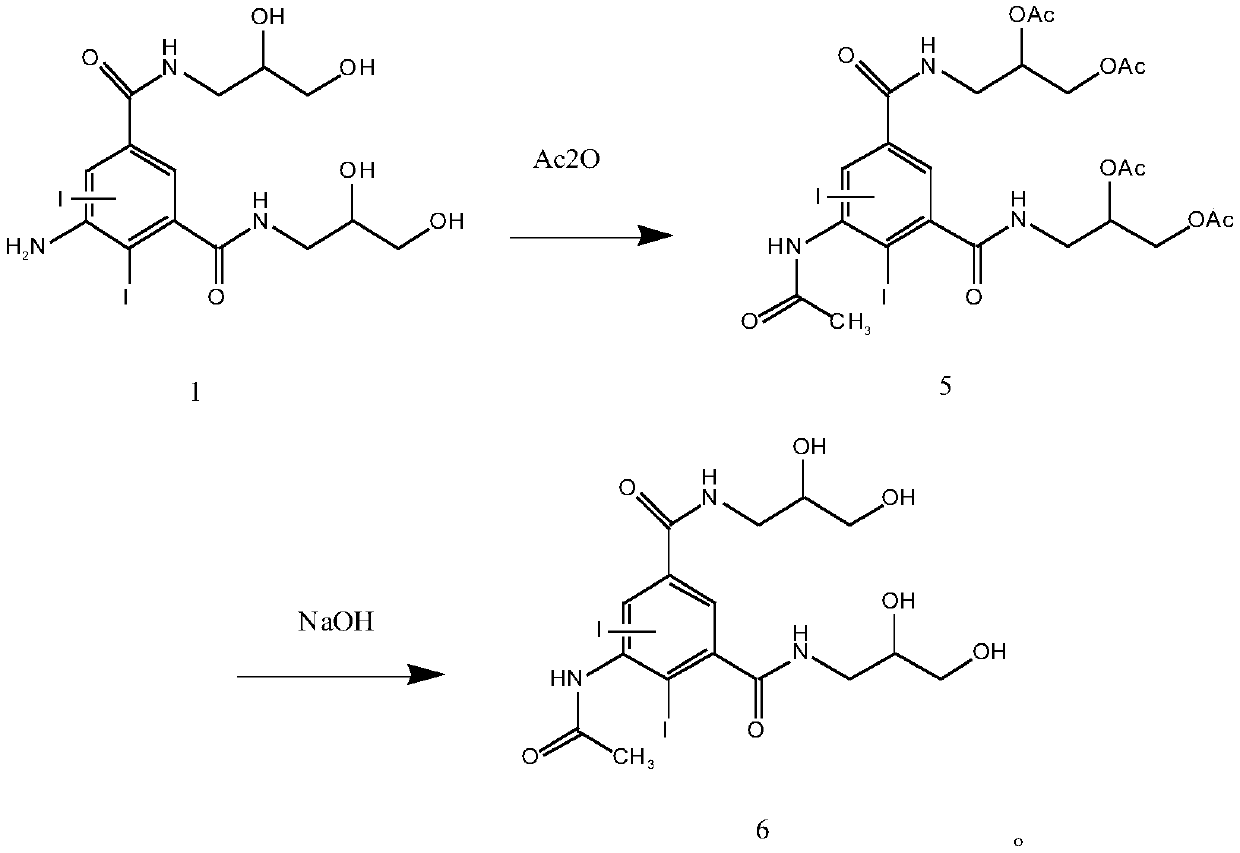
[0041] Embodiment 2: the synthesis of iohexol impurity G (compound of formula 6)
[0042] According to formula 1 compound and acetic anhydride molar ratio 1:4, add 34.3g acetic anhydride, concentrated sulfuric acid (mass concentration 70% H 2 SO 4 Aqueous solution) 130mL, 50g compound of formula 1, stir evenly and heat up to 50-100°C, react for 10-20 hours, HPLC detects that the reaction is complete, evaporate unreacted acetic anhydride, add 100mL of water, at room temperature, add an appropriate amount of hydroxide Sodium, adjust the pH to 9-11, HPLC detects the completion of the reaction, adjust the pH to 5-7 with hydrochloric acid, cool down to 0-15°C and crystallize to obtain 5-acetylamino-N,N'-bis(2,3-di Hydroxypropyl)-2,4,6-triiodo-1,3-phthalamide solid (compound of formula 6), purity 97.2%.
Embodiment 3
[0043] Embodiment 3: the synthesis of iohexol impurity H (compound of formula 7)
[0044]According to formula 6 compound and 3-chloro-1,2-propanediol, the molar ratio of sodium hydroxide is 1:1:1, add 20g compound formula 6, ethylene glycol monomethyl ether 60mL, sodium hydroxide 1.25g, 3-Chloro-1,2-propanediol 3.45g, heat to 30-100°C for 10-20 hours, distill out ethylene glycol monomethyl ether, add 30mL of water, adjust the pH to 5-7 with hydrochloric acid, add isopropanol 60mL, cooled to 5-10°C, precipitated crystals, dried to obtain 5-[acetyl(2,3-dihydroxypropyl)amino]-N,N′-bis(2,3-dihydroxypropyl)-2 , 4,6-triiodo-1,3-benzenedicarboxamide solid (compound of formula 7), purity 96.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com