Fluorescent probe for detecting long wave emission of benzenethiol
A fluorescent probe, thiophenol technology, applied in the field of fluorescent probes, to achieve the effects of eliminating background interference, good selectivity, and strong tissue penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 compound I
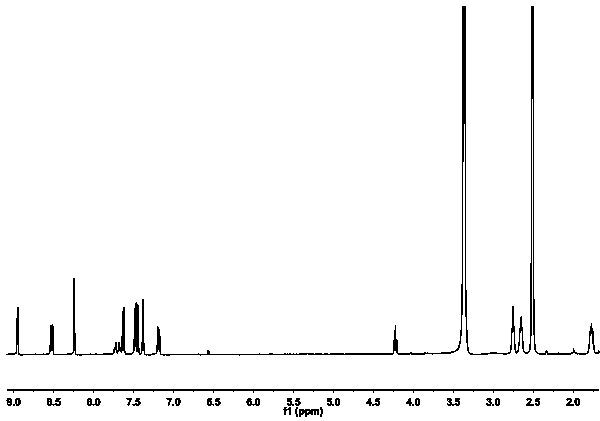
[0027] Under the condition of nitrogen protection and ice bath, compound II was dissolved in anhydrous dichloromethane, then 2,4-dinitrofluorobenzene was added, and triethylamine was added dropwise into the reaction system. Stir overnight at room temperature. The crude product was obtained, which was purified by column chromatography to obtain red compound I. figure 1 is the NMR image of the probe.
Embodiment 2
[0028] Embodiment 2 Probe compound I selectivity analysis
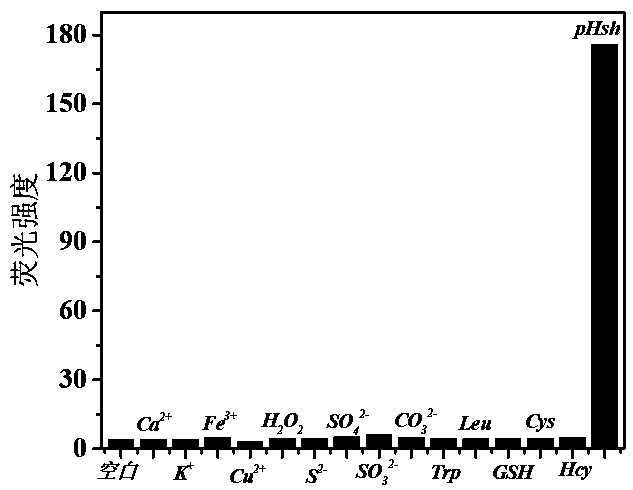
[0029] Add 10 μM compound I (PBS 20 mM, pH = 7.4) to the DMF and PBS buffer containing thiophenol at a volume ratio of 2:8, and the detection results are as follows: figure 2 , when the excitation light wavelength is 560 nm, the probe compound I has a strong fluorescence response to thiophenol at 645 nm, and the probe compound I has no obvious response to other interference ions, indicating that the probe compound I has a strong fluorescence response to thiophenol Has excellent selectivity.
Embodiment 3
[0030] Example 3 Analysis of the response of probe compound I to changes in the concentration of thiophenol
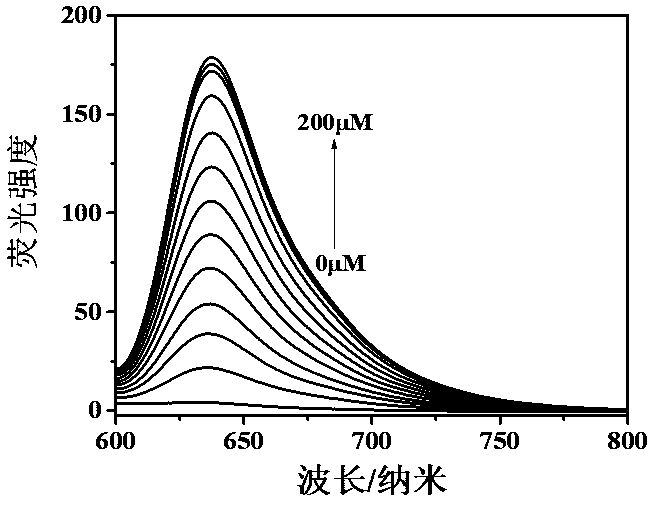
[0031] Add 10 μM probe compound I to the buffer containing different concentrations of thiophenol (0 - 200 μM), the fluorescence response intensity increases linearly with the increase of the amount of thiophenol added, and the detection results are as follows: image 3 , the results show that the probe compound I has a wider detection range and higher sensitivity to the concentration of thiophenol. The working curve was made with the coordinates of the maximum value of the fluorescence intensity and the corresponding concentration of thiophenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com