Immune cell containing tumor antigen recognition receptor and application of immune cell
A technology of immune cells and tumor antigens, applied in receptors/cell surface antigens/cell surface determinants, medical preparations containing active ingredients, applications, etc. Difficulty secreting T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1. Design of CAR / TCR sequence
[0051] The proteins involved in the present invention include PH20, CD8α hinge region, human CD8α transmembrane region, human 4-1BB intracellular region and human CD3ζ intracellular region, MSLN, HAS2 (hyaluronidase synthesis gene) and targeting each Target scFv sequence, TCR sequence. The NCBI accession number of PH20 amino acid sequence is NP_694859.1 (cDNA is NM_153189.2); the NCBI accession number of HAS2 is NP_005319.1 (cDNA is NM_005328.3); the NCBI accession number of MSLN is NP_037536.2 (cDNA is NM_013404 .4); tEGFR is the III and IV domains of EGFR, and the NCBI accession number of EGFR is NP_005219.2 (cDNA is NM_005228.4). The amino acid sequence and coding base sequence of MSLN scFv (SS1) are derived from patent US7977457 (SEQ ID NO 12); the amino acid sequence of GPC3 scFv is derived from patent CN104140974A (SEQ ID NO 22), and the base sequence is obtained by codon optimization for human ; The amino acid sequence an...
Embodiment 2
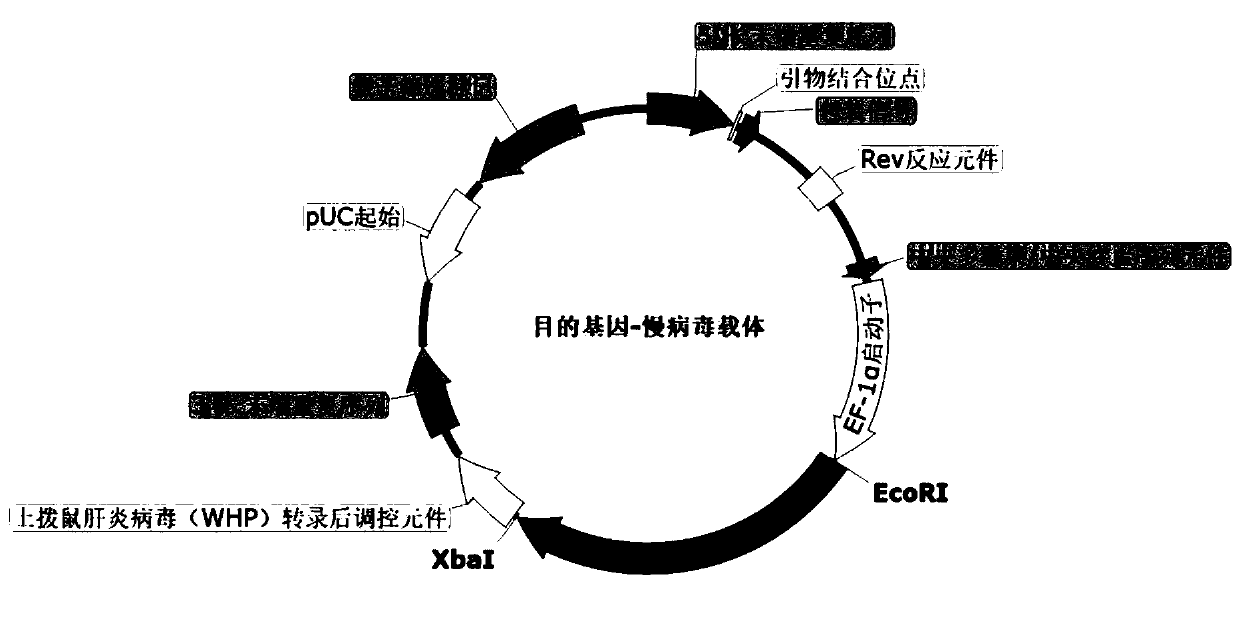
[0066] Example 2. Vector Construction
[0067] The base sequences of PH20 and CAR / TCR synthesized in Example 1 were digested with EcoRI and XbaI, connected with DNALigation Kit (Takara) and inserted into the corresponding sites of the lentiviral vector pCDH-EF1a (SBI) ( figure 1 ), to obtain the target gene expression vector Lenti-GOI. The base sequence of MSLN was digested with BamHI and EcoRI, and inserted into the corresponding site of pcDNA3.1 vector (Invitrogen) by DNA Ligation Kit (Takara); the base sequence of HAS2 was digested by NheI and BamHI, and inserted into pcDNA3 by DNA Ligation Kit. . Corresponding sites of 1-Hyg vector (Invitrogen). The ligation products were transformed into competent Escherichia coli (Top10). On the second day after transformation, the clones were picked and cultured in LB liquid medium containing 100 μg / mL ampicillin (Saigon Biotechnology), and sequenced for identification. The clones with correct sequencing results were selected and ino...
Embodiment 3
[0070] Example 3. Lentiviral packaging
[0071] Prepare a 10cm cell culture dish and inoculate 5×10 6 293 cells / dish, add complete medium DMEM high glucose (GIBCO) + 10% FBS (GIBCO), place at 37°C, 5% CO 2 incubator, overnight.
[0072] Take out 1 mg / ml polyetherimide (PEI, Polyscience) transfection reagent and lentiviral packaging plasmid (Lenti-GOI, pMD2.G, psPAX2), and thaw at room temperature. Remove the HBSS buffer and warm to room temperature. Take 2 mL of PBS to one well of a 6-well plate, add 10 μg Lenti-GOI, 7 μg psPAX2, 4 μg pMD2.G and mix well, add 63 μL PEI transfection reagent and mix well with a pipette, and let stand at room temperature for 10 minutes. Add the DNA / PEI complex drop by drop to a 10cm Petri dish, shake the Petri dish gently, and mix well. Place the Petri dish at 37°C, 5% CO 2 Incubator, after culturing for 6-8 hours, remove the medium containing the transfection reagent, replace it with fresh complete medium and put it back into the incubator ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com