Preparation and activity identification method of a pig-derived second messenger molecule 2′3′-cgamp
An active, porcine-derived technology, applied in the field of preparation and activity identification of porcine-derived second messenger molecule 2'3'-cGAMP, can solve the problems of limited wide application, difficult separation and purification, complicated process, etc. Low cost, broad antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
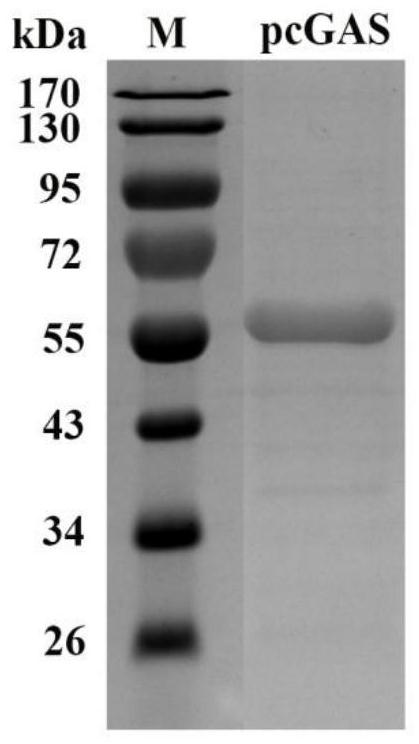
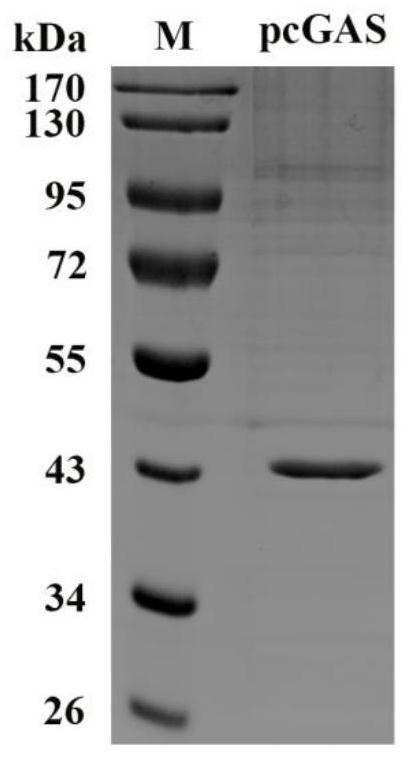
[0064] Example 1. Preparation of recombinant pig-derived 2′3′-cGAMP synthetase pcGAS
[0065] The amino acid sequence of the pcGAS enzyme (porcine cyclic guanylate-adenylate synthetase) is shown in Sequence 1 of the Sequence Listing, wherein the active catalytic domain of the enzyme is at positions 136-495 from the N-terminus. The coding gene of the pcGAS enzyme is shown in Sequence 2 of the Sequence Listing, wherein the enzyme activity catalytic domain is encoded from the 406-1488 position at the 5' end.
[0066] 1. Replace the fragment between the BamH I and Hind III sites of the prokaryotic expression vector pET-28a-SUMO (Wuhan Miaoling Biotechnology Co., Ltd., article number: P0028) with sequence 2 from the 5' end 406- The DNA molecule indicated at position 1488 was used to obtain the recombinant expression vector pET-28a-SUMO-pcGAS (sequencing verification).
[0067] 2. Introduce the recombinant expression vector pET-28a-SUMO-pcGAS obtained in step 1 into Escherichia col...
Embodiment 2
[0075] Example 2. Using recombinant pcGAS enzyme to catalyze the synthesis of pig-derived 2'3'-cGAMP
[0076] Using the pig-derived recombinant pcGAS enzyme prepared in Example 1 to efficiently and specifically catalyze the synthesis of pig-derived 2′3′-cGAMP in vitro, the details are as follows:
[0077] 1. Configure the enzymatic reaction system (as shown in Table 1). The enzymatic reaction system was configured on ice.
[0078] Table 1 Enzymatic reaction system
[0079]
[0080] 2. After completing step 1, incubate at 37°C for 2 hours, then add 50 μL of nuclease Benzonase, and incubate at 37°C for 30 minutes.
[0081] 3. After completing step 2, incubate the reaction product at 95°C for 10 minutes, then centrifuge at 16000g / min for 10 minutes, and take the supernatant. The supernatant was filtered with a 0.5ml / 10kDa ultrafiltration centrifuge tube (Merck-Millipore, catalog number: UFC501096) to obtain the reaction product.
[0082] The absorbance value of the reaction ...
Embodiment 3
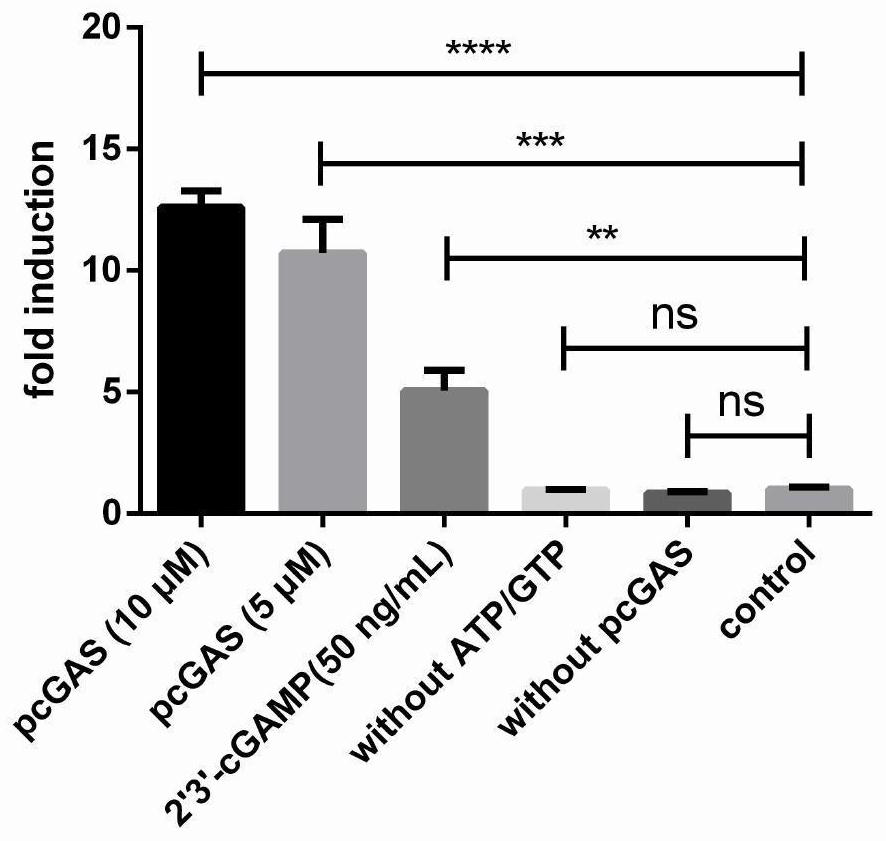
[0084] Example 3: Activity identification of pig-derived pcGAS and catalytic product 2′3′-cGAMP
[0085] RAW264.7-Lucia TM ISG cells are a reporter cell in which the expression of luciferase gene (Lucia luciferase gene) is jointly driven by the ISG54 promoter and the interferon stimulation response element ISRE. When stimulated by 2′3′-cGAMP, by RAW264.7-Lucia TM The STING molecule expressed by ISG cells recognizes and activates the transcription factors IRF3 and IRF7, and then combines with ISRE to induce the secretion of luciferase gene, and then through QUANTI-Luc TM The fluorescence value of luciferase was detected to reflect the enzymatic activity of pcGAS and the stimulating activity of 2′3′-cGAMP.
[0086] 1. Connect RAW264.7-Lucia TM ISG cells were adjusted to a density of 5 × 10 5 / mL, seeded in a 96-well plate (100 μL per well, the number of cells is 5×10 4 / mL), cultured at 37°C until the cell density reached 70%-80%.
[0087] 2. After completing step 1, ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com