Polypeptide microsphere with effects of quick release and slow release and preparation method of polypeptide microsphere
A mild and microsphere technology, which is applied in the field of polypeptide microspheres and its preparation, can solve the problems of slow onset of action, low release, and major safety issues, and achieve the effects of good shape, maintaining blood drug concentration, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
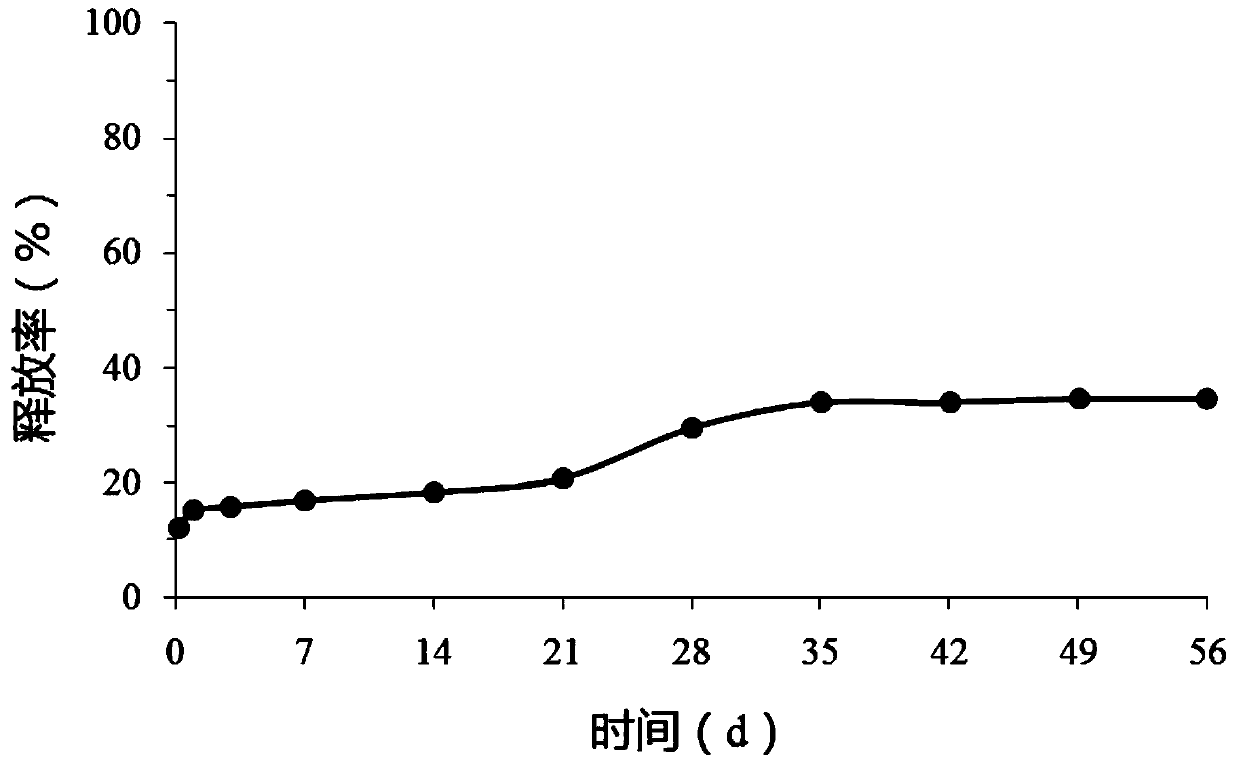
[0039] Weigh 15 mg of triptorelin acetate and 2 mg of Tween 80 and co-lyophilize, the cold trap temperature is -50°C, and the lyophilization pressure is 0.52 mbar. Weigh 500 mg of PLGA and dissolve it in 20 g of dichloromethane, and directly disperse the obtained co-lyophilized product in the dichloromethane solution of PLGA. After ultrasonic dispersion, stir at a speed of 1000 rpm and add 12.5 g of simethicone, so that PLGA is wrapped on the surface of triptorelin acetate. The resulting mixture was then added to a 750 mL n-heptane bath, solidified at a rotation speed of 400 rpm, and solidified for 30 min. Stand still, and after the suspended matter settles to the bottom, remove the upper layer to clarify n-heptane, wash with petroleum ether three times, dry, and collect the polypeptide microspheres.
Embodiment 2
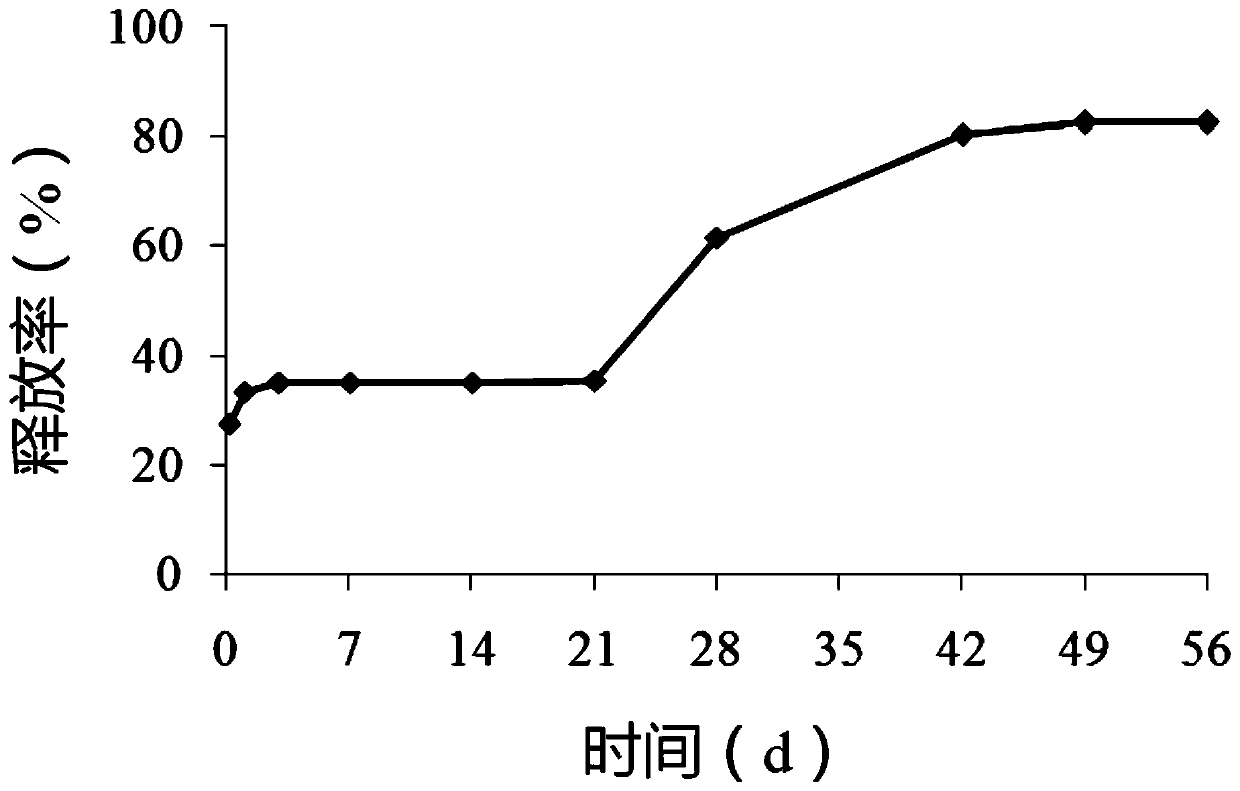
[0041] Weigh 15 mg of triptorelin acetate and 4 mg of Tween 80 and co-lyophilize, the cold trap temperature is -50°C, and the lyophilization pressure is 0.52 mbar. Weigh 500 mg of PLGA and dissolve it in 20 g of dichloromethane, and disperse the co-lyophilized product directly in the dichloromethane solution of PLGA. After ultrasonic dispersion, stir at a speed of 1000 rpm and add 12.5 g of simethicone, and then dissolve the obtained The mixture was added to a 750mL n-heptane bath, solidified at a speed of 400rpm, and solidified for 30min. Stand still, and after the suspended matter settles to the bottom, remove the upper layer to clarify n-heptane, wash with petroleum ether three times, dry, and collect the polypeptide microspheres.
Embodiment 3
[0043] Weigh 40mg of exenatide and 2mg of Tween 20 and co-lyophilize, the temperature of the cold trap is -60°C, and the lyophilization pressure is 0.37mbar. Weigh 400 mg of PLA and dissolve it in 20 g of ethyl acetate, and disperse the co-lyophilized product directly in the PLA ethyl acetate solution. After ultrasonic dispersion, stir at a speed of 1000 rpm and add 12.5 g of simethicone, and then dissolve the obtained The mixture was added to a 750mL n-heptane bath, solidified at a speed of 400rpm, and solidified for 30min. Stand still, and after the suspended matter settles to the bottom, remove the upper layer to clarify n-heptane, wash with petroleum ether three times, dry, and collect the polypeptide microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com