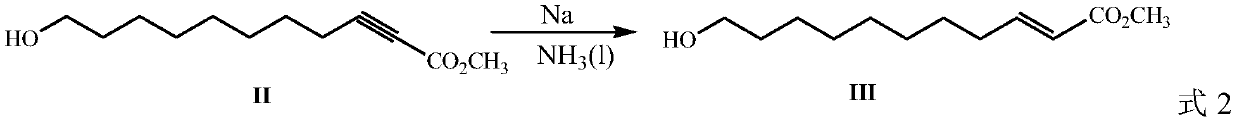Method for preparing royal jelly acid
A technology of royal jelly acid and methyl propiolate, applied in carboxylate preparation, organic chemistry, etc., can solve the problems of high requirements for reaction conditions, high production costs, hindering the progress of the reaction, etc., and achieves easy industrial production and short reaction route , the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
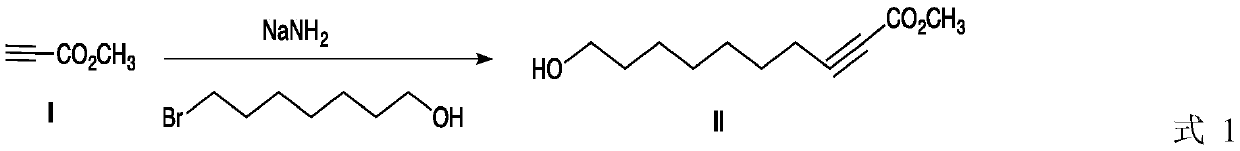
[0028] Add 8.4 g (0.1 mol) methyl propiolate, 30 mL dry tetrahydrofuran and 4.5 g (0.115 mol) sodium amide in a 100 mL single-necked bottle, stir the mixture at room temperature for about 15 minutes, then add 7-bromoheptanol dropwise 21.34 g (0.11 mol), continued to react for 3.0 h after 0.5 h of dropwise addition. After the reaction is complete, remove tetrahydrofuran in vacuo, dissolve the residue with dichloromethane, wash with water, separate the organic phase, anhydrous MgSO 4 After drying and filtering, the filtrate was evaporated to remove the solvent to obtain 19.2 g of compound II as a light yellow oily liquid, with a yield of 97%.
[0029] 1 HNMR (CDCl 3 ,δ(ppm),TMS):δ1.26-1.44(m,8H),1.49-1.58(m,2H),2.46(t,2H),3.62(t,2H),3.68(s,3H)4.08 (s,br,1H).
[0030] In a 100 mL single-necked flask at -40°C, 20 mL of liquid ammonia was passed, then 2.4 g (0.104 mol) of sodium metal was added, and 9.9 g (0.05 mol) of compound II prepared above was added, and the reaction was ...
Embodiment 2
[0035] Add 8.4 g (0.1 mol) methyl propiolate, 30 mL dry tetrahydrofuran and 4.1 g (0.105 mol) sodium amide into a 100 mL single-necked bottle, stir the mixture at room temperature for about 15 minutes, then add 7-bromoheptanol dropwise 20.37 g (0.105 mol), continued to react for 3.0 h after 0.5 h of dropwise addition. After the reaction is complete, remove tetrahydrofuran in vacuo, dissolve the residue with dichloromethane, wash with water, separate the organic phase, anhydrous MgSO 4 After drying and filtering, the filtrate was evaporated to remove the solvent to obtain 19.1 g of compound II as a light yellow oily liquid, with a yield of 96.5%.
[0036] In a 100 mL single-necked flask at -40°C, 20 mL of liquid ammonia was passed, then 2.5 g (0.108 mol) of sodium metal was added, and 9.9 g (0.05 mol) of compound II prepared above was added, and the reaction was stirred at low temperature for 2 h. After the reaction was completed, the liquid ammonia was removed by filtration, ...
Embodiment 3
[0039] Add 25.2 g (0.3 mol) methyl propiolate, 100 mL dry tetrahydrofuran and 12.3 g (0.315 mol) sodium amide in a 250 mL three-necked flask, stir the mixture at room temperature for about 30 minutes, then add 7-bromoheptanol dropwise 63.15 grams (0.326mol), after 1.0 hours of dropwise addition, the reaction was continued for 3.5 hours. After the reaction is complete, remove tetrahydrofuran in vacuo, dissolve the residue with dichloromethane, wash with water, separate the organic phase, anhydrous MgSO 4 After drying, filtering, and evaporating the filtrate to remove the solvent, 57.5 g of Compound II was obtained as a pale yellow oily liquid, with a yield of 96.8%.
[0040] 100 ml of liquid ammonia was passed into a 2050 mL single-necked flask at -40°C, then 10.3 g (0.445 mol) of sodium metal was added, and 40.2 g (0.203 mol) of compound II prepared above was added, and the reaction was stirred at low temperature for 2.5 h. After the reaction was completed, the liquid ammonia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com